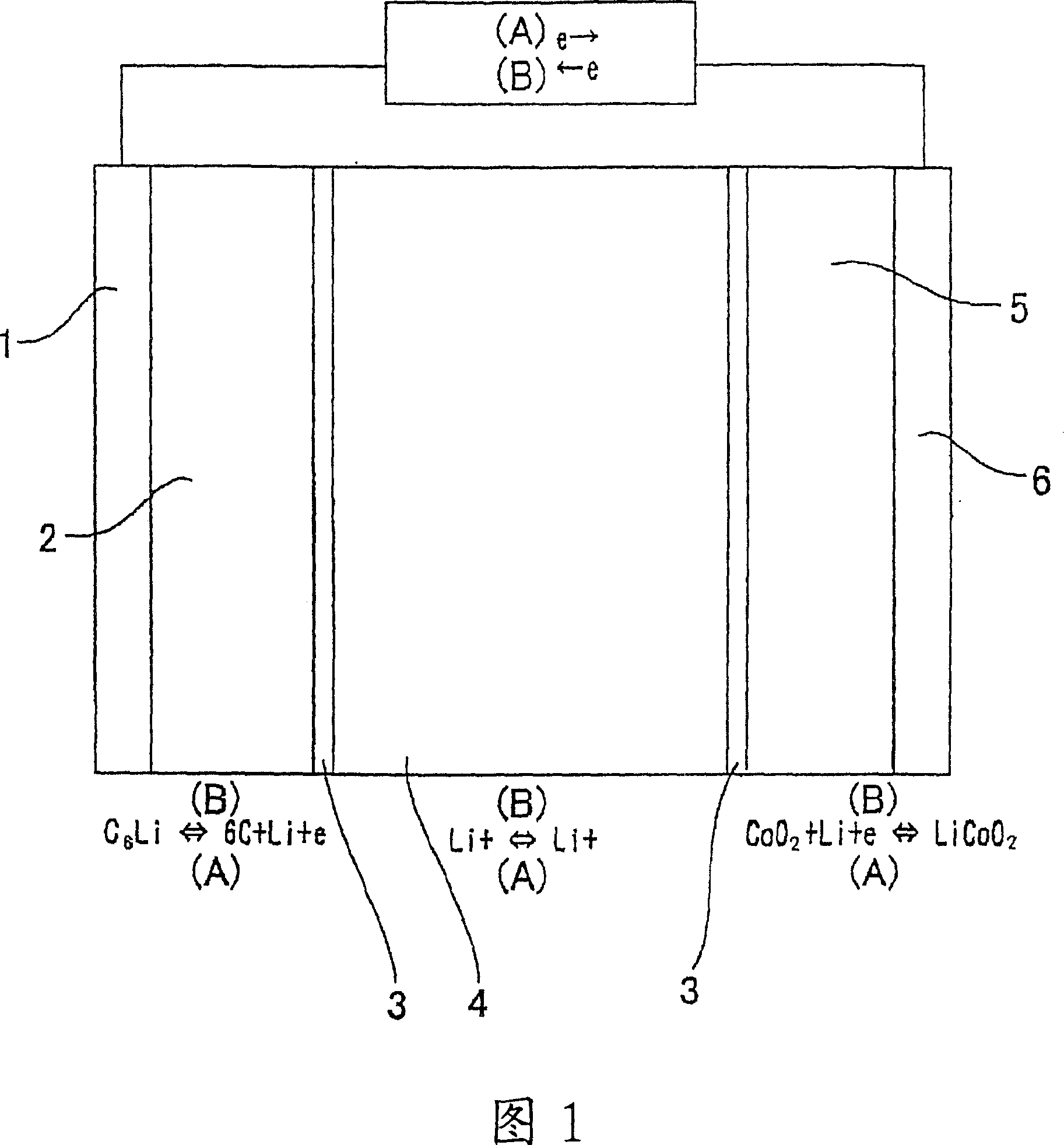
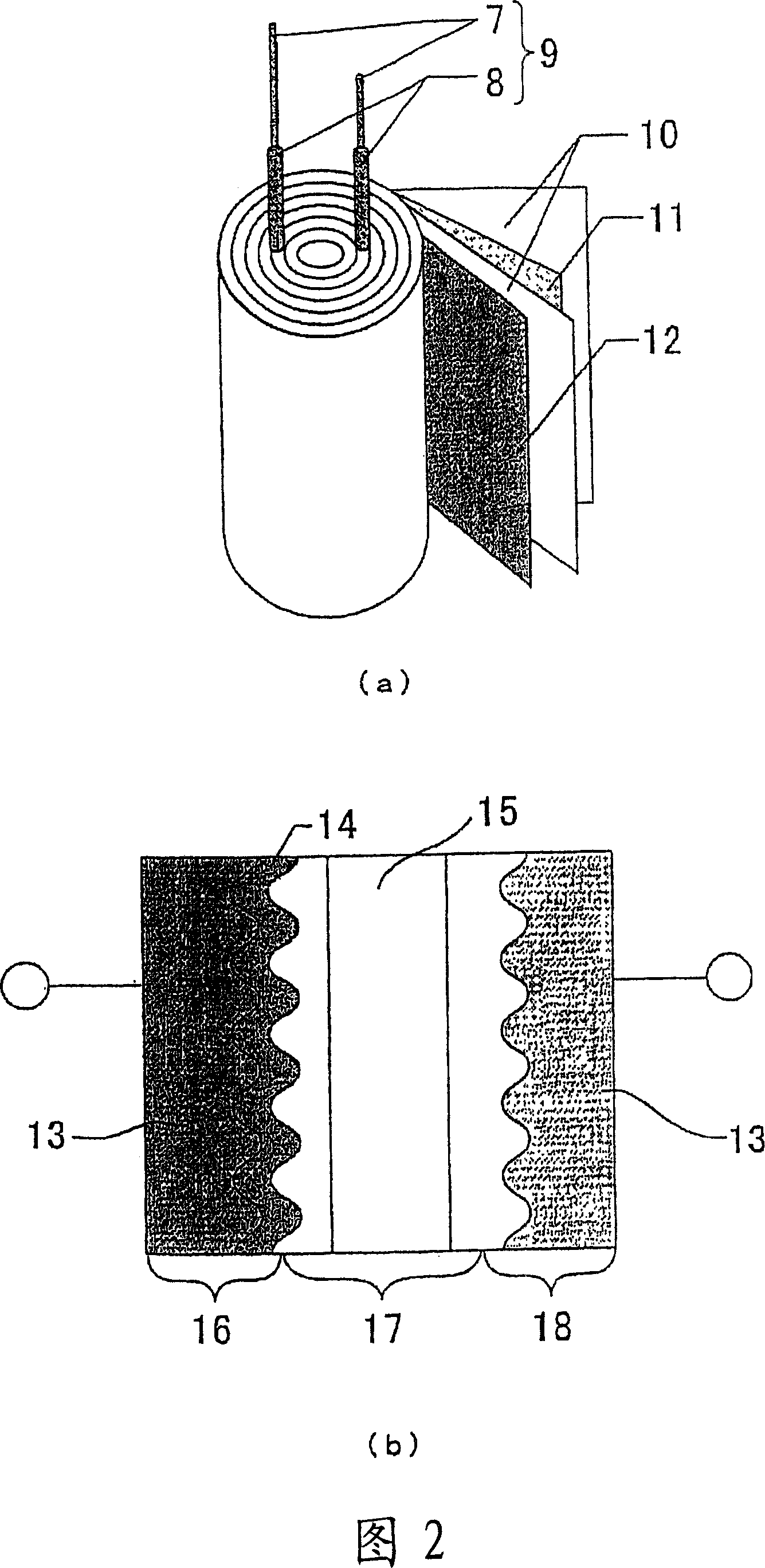
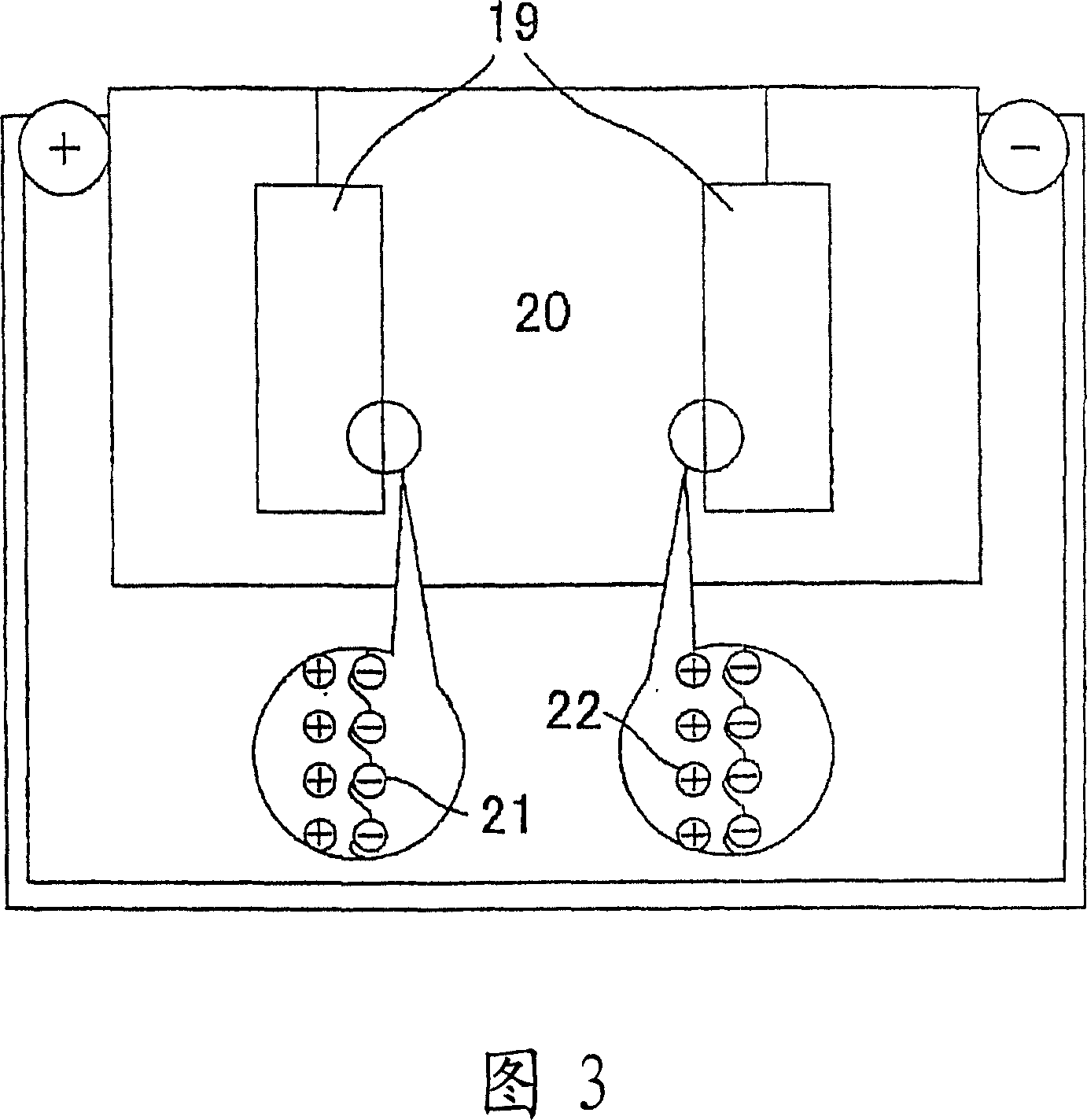
Material for electrolytic solution, ionic material-containing composition and use thereof
A technology of ionic materials and electrolytes, applied in the field of electrolyte materials, can solve the problems of undisclosed performance parameters, insufficient ionic conductivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0334] 1% by mass of ion-exchanged water was mixed into ethylmethylimidazolium dicyanamide to prepare an ion-conductive material. The ionic conductivity of the ion-conducting material was measured, and its ionic conductivity was 2.6×10 -2 S / cm(25℃), 1.3×10 -2 S / cm(0℃), 5.5×10 -3 S / cm(-20℃) and 2.7×10 -6 S / cm (-55°C). The results are shown in Table 1-1.
[0335] Using an impedance analyzer of SI1260 (trade name, product of Solartron Co., Ltd., in which a SUS electrode is used), the above ion conductivity was measured by a complex impedance method.
Embodiment 2~19 and comparative example 1~4
[0337] According to the same method as in Example 1, except for using the kinds and amounts of the ionic liquids and solvents shown in Table 1-1, the ion conductivity was measured. The results obtained are shown in Table 1-1.
[0338]
Ionic conductivity (S / cm)
type
quality%
25℃
0℃
-20℃
-55℃
Example 1
EMImDCA
ion exchange water
1
2.6×10 -2
1.3×10 -2
5.5×10 -3
2.7×10 -5
Example 2
EMImDCA
ion exchange water
10
3.4×10 -2
2.2×10 -2
1.1×10 -2
1.2×10 -3
Example 3
EMImDCA
GBL
1
2.3×10 -2
1.1×10 -2
4.5×10 -3
5.8×10 -7
Example 4
EMImDCA
GBL
10
2.6×10 -2
1.1×10 -2
4.5×10 -3
5.2×10 -5
Example 5
EMImDCA
GBL
50
7.4×10 -2
4.4×10 -2
2.4×10 -2
4.2×10 -3
Example ...
Embodiment A
[0349] Ethylmethylimidazolium tricyanomethyl (EMImTCM) was dissolved in the mobile phase at 2% by mass, and the peak decrease rate after the heat resistance test was measured by LC (liquid chromatography) analysis. The results are shown in Table 1-2. In addition, the heat resistance test and LC analysis were performed under the following conditions. The peak reduction rate was measured in the above-mentioned manner.
[0350] (heat resistance test)
[0351] The sample was left at 150° C. for 50 hours using a drier called DNF-400 (trade name; manufactured by Yamato Scientific Co., Ltd.).
[0352] (LC analysis)
[0353] Measuring instrument: Manufactured by Tosoh Corporation
[0354] Detector: UV-8020 (UV absorption 254nm)
[0355] Mobile phase: 10% methanol in water
[0356] Current intensity (Current): 1.0 moles
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ionic conductivity | aaaaa | aaaaa |
| Ionic conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com