Gene expression product of extro-cellular domain amino end of human thyrotropin receptor, its preparing method and application in enzyme immune technology
A technology of thyrotropin and expression products, applied in the field of genetic engineering, can solve the problems of only measuring TRAb, unsatisfactory expression yield, and inability to distinguish TSAb and TSBAb, etc., and achieve the effect of method sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0056] 1. Cloning of human thyrotropin receptor extracellular region amino terminal, middle segment and carboxy terminal gene, construction and identification of expression plasmid
[0057] (1) Cloning of amino-terminal, middle segment and carboxyl-terminal genes of extracellular region of human thyrotropin receptor
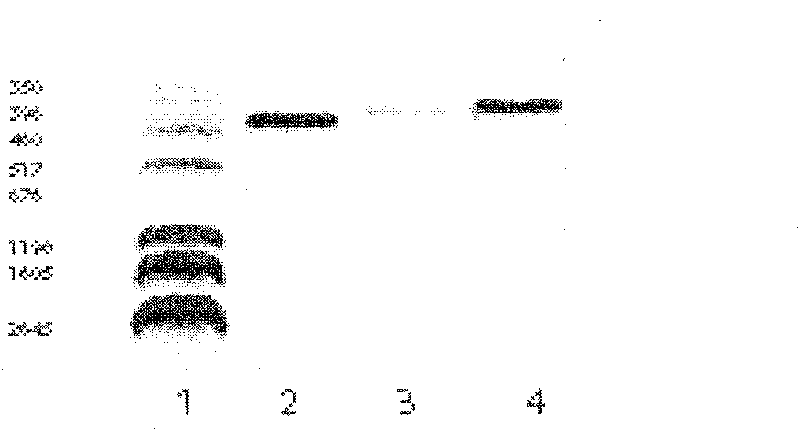
[0058] The first strand of human thyroid cDNA was synthesized by reverse transcription of total RNA extracted from the excised thyroid tissue of patients with Graves' disease by TRIzol one-step method, and the hTSHR-N terminal and hTSH-C were amplified by RT-PCR with self-designed primers end and hTSHR-M encoding gene. Taking the nucleic acid molecular weight standard as a reference, identify the PCR amplification product with 1.5% agarose gel electrophoresis, and the results show that a clear and specific electrophoresis band can be seen at 462bp, 417bp and 405bp respectively, which is consistent with the expected gene fragment size (see figure 1 ). figure 1 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com