Nano iron oxide catalyst and its synthetic method
A technology of nano-iron oxide and synthesis method, which is applied in the direction of physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, etc. Slow activity, poor thermal stability and other problems, to achieve the effect of superior oxidation catalytic performance, low cost, and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
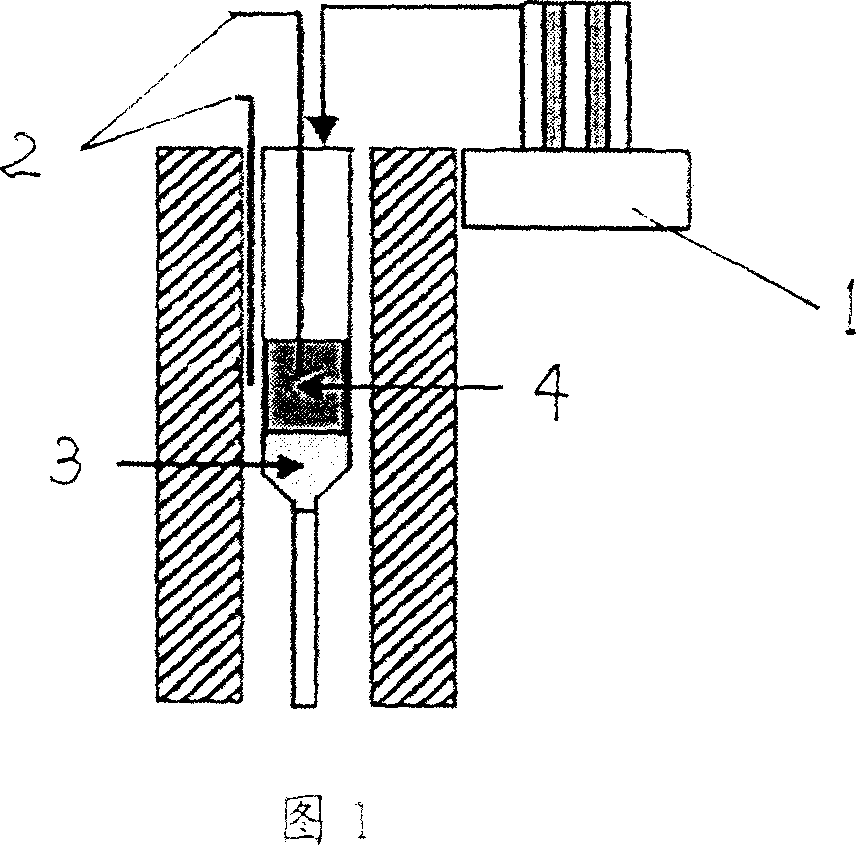
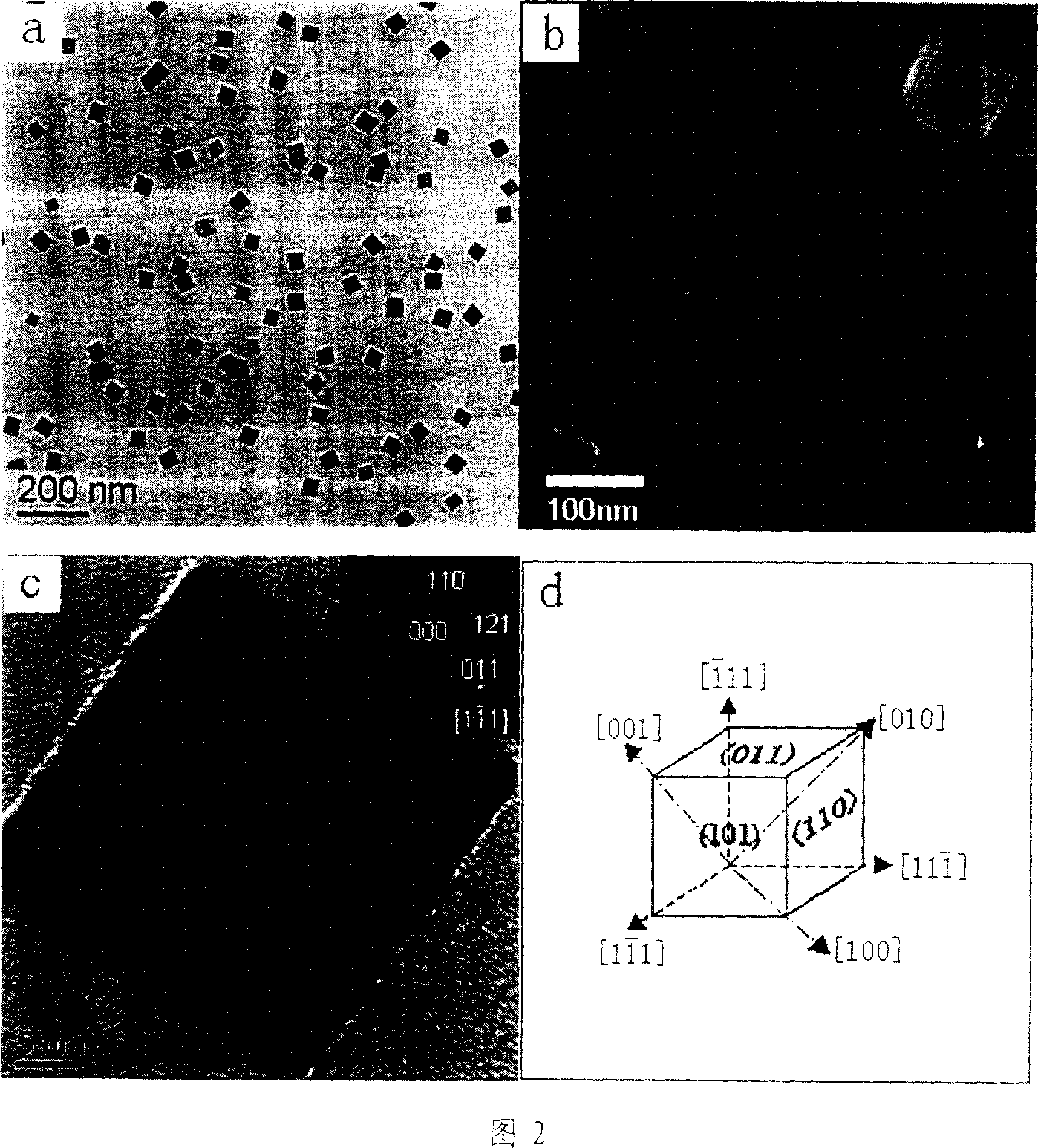
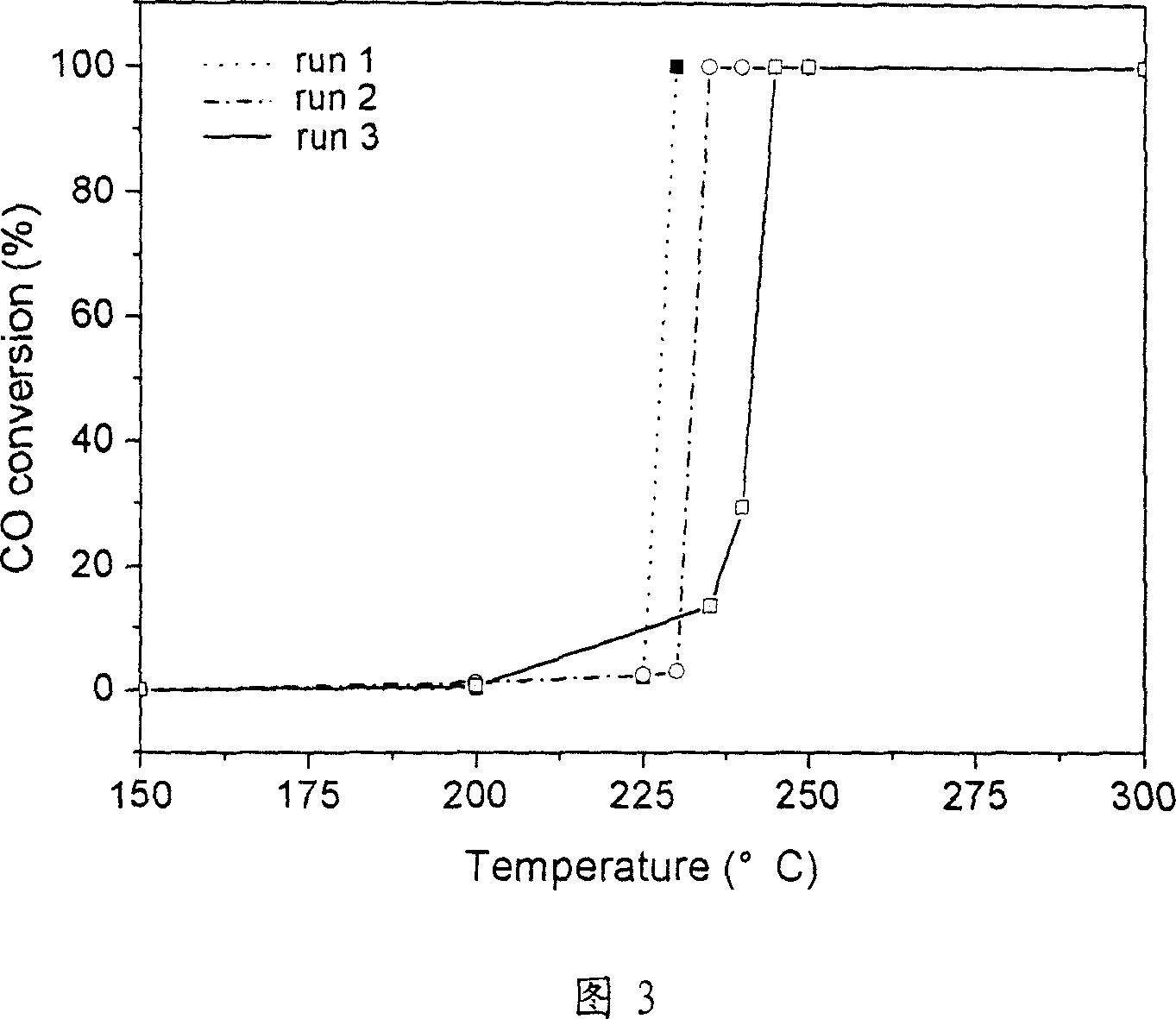
Image
Examples
example 1
[0011] Example 1: 0.404g (1mmol) analytically pure ferric nitrate and 0.600g molecular weight are 30000 polyvinylpyrrolidone surfactant (PVP, Mw=30000) to add in the 70ml polytetrafluoroethylene hydrothermal tank , then accurately measure 36ml of N,N-dimethylformamide (DMF) with a pipette and stir thoroughly to obtain a transparent dark red solution. Then seal the hydrothermal tank with a steel shell, turn it into a resistance furnace and heat it up to 160°C at a heating rate of 5°C / min and keep it warm for 10 hours, then continue to heat it up to 180°C at a heating rate of 5°C / min and keep it warm for 20 hours. After the reaction, the hydrothermal tank was naturally cooled to room temperature. Finally, the red precipitate was washed with water and ethanol, centrifuged, and dried naturally in air to obtain a reddish-brown iron oxide powder catalyst.
example 2
[0012] Example 2: 0.270g (1mmol) ferric chloride and 0.600g molecular weight are the polyvinylpyrrolidone surfactant (PVP, Mw=30000) of 30000 to add in the 70ml polytetrafluoroethylene hydrothermal tank, then accurately measure with pipette Take 36ml of N,N-dimethylformamide (DMF) and stir well to obtain a transparent dark red solution. Then seal the hydrothermal tank with a steel shell, turn it into a resistance furnace and heat it up to 160°C at a heating rate of 5°C / min and keep it warm for 10 hours, then continue to heat it up to 180°C at a heating rate of 5°C / min and keep it warm for 20 hours. After the reaction, the hydrothermal tank was naturally cooled to room temperature. Finally, the red precipitate was washed with water and ethanol, centrifuged, and dried naturally in air to obtain a reddish-brown iron oxide powder catalyst.
example 3
[0013] Example 3: 0.199g (1mmol) ferrous chloride and 1.160g molecular weight are the polyvinylpyrrolidone surfactant (PVP, Mw=58000) of 58000 and add in the 70ml polytetrafluoroethylene hydrothermal jar, then accurately with pipette Measure 36ml of N,N-dimethylformamide (DMF) and stir thoroughly to obtain a transparent dark red solution. Then seal the water-heating tank with a steel shell, turn it into a resistance furnace and raise the temperature to 180°C at 5°C / min and keep it warm for 30h. After the reaction, the hydrothermal tank was naturally cooled to room temperature. Finally, the red precipitate was washed with water and ethanol, centrifuged, and dried naturally in the air to obtain a reddish-brown iron oxide powder catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com