Glomerulus-targeted protein nanoparticle pharmaceutical composition and application thereof
A nanoparticle and glomerulus technology, which is applied in the field of medicine, can solve the problems of cumbersome preparation process, in vivo toxicity, cumbersome and complicated methods, etc., and achieve the effect of simple and easy preparation technology, reducing toxic and side effects, and improving therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
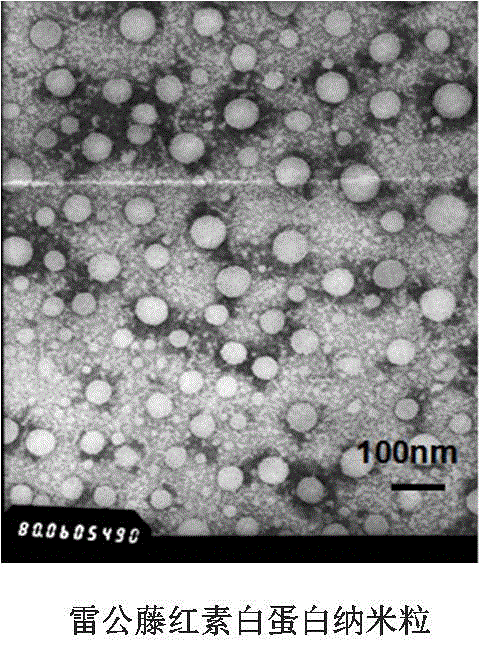
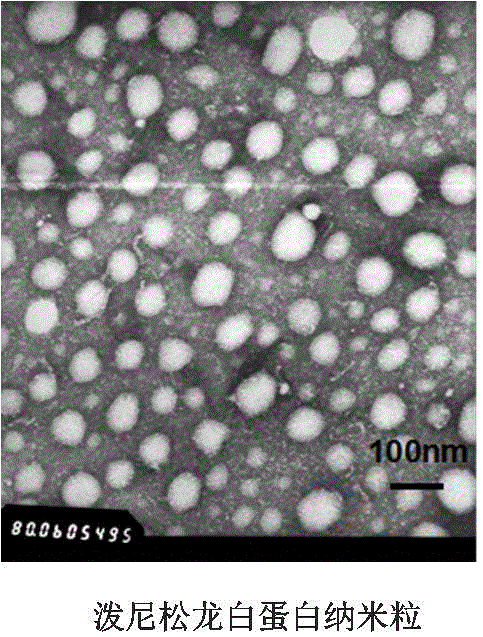
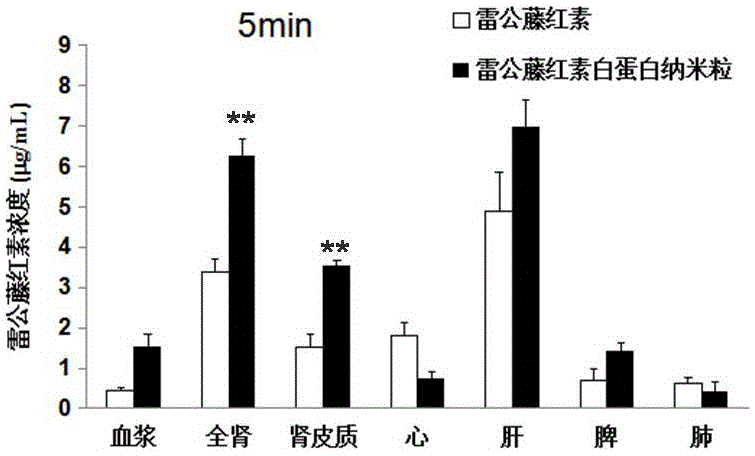
[0078] Preparation and characterization of human serum albumin nanoparticle tripterine composition and human serum albumin nanoparticle prednisolone composition by ultrasonic method
[0079] Dissolve tripterine 7mg or prednisolone 20mg and water-insoluble stabilizer soybean oil 50mg in 2mL dichloromethane:ethyl acetate (7:3, V / V), and then distill with 10mL human serum albumin Mix the aqueous solution (2%W / V), in an ice bath, ultrasonic power 500W (3s / 5s) for 8min, and then remove the organic mixed solvent by rotary evaporation under reduced pressure at 37°C to obtain human serum albumin nanoparticles tripterine composition and human serum albumin nanoparticle prednisolone composition. The particle size and potential were measured by a Malvern particle sizer, the morphology was characterized by a transmission electron microscope, and the encapsulation efficiency was determined by a Sephadex column method.
[0080] result:
[0081] As shown in Table 1, the prepared human seru...
Embodiment 2
[0085] Preparation of human serum albumin nanoparticle tripterine composition and human serum albumin nanoparticle prednisolone composition by high pressure homogenization method
[0086] Dissolve tripterine 7mg or prednisolone 20mg in 2mL dichloromethane:ethyl acetate (7:3, V / V), and then mix with 10mL human serum albumin (2%W / V) and water-soluble Solutol HS-15 40mg double-distilled aqueous solution was mixed, and the probe was ultrasonicated 15 times in an ice bath, and the obtained suspension was transferred to a high-pressure homogenizer, and the nanoemulsion was obtained by circulating and homogenizing 15 times in a pressure range of 15000Psi. Under reduced pressure at 37°C, the organic mixed solvent was removed by rotary evaporation to obtain the human serum albumin nanoparticle tripterine composition and the human serum albumin nanoparticle prednisolone composition, with an average particle size of about 100nm and a PDI Both are below 0.2.
Embodiment 3
[0088] Preparation of human serum albumin nanoparticle tripterine composition and human serum albumin nanoparticle prednisolone composition by microfluidic method
[0089] Dissolve tripterine 7mg or prednisolone 20mg and water-insoluble stabilizer medium-chain oil 50mg in 2mL dichloromethane:ethyl acetate (7:3, V / V), and then mix with 10mL human serum albumin (2 %W / V) and water-soluble stabilizer Solutol HS-15 40mg double-distilled aqueous solution mixed, the probe was ultrasonicated 15 times in an ice bath, the obtained suspension was transferred to a high-pressure homogenizer, and circulated within a pressure range of 20000Psi The nanoemulsion was obtained after 15 times of melting, and then the organic mixed solvent was removed by rotary evaporation under reduced pressure at 37°C, and the human serum albumin nanoparticle tripterine composition and the human serum albumin nanoparticle prednisolone composition were obtained. The average particle size is around 90nm, and the P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com