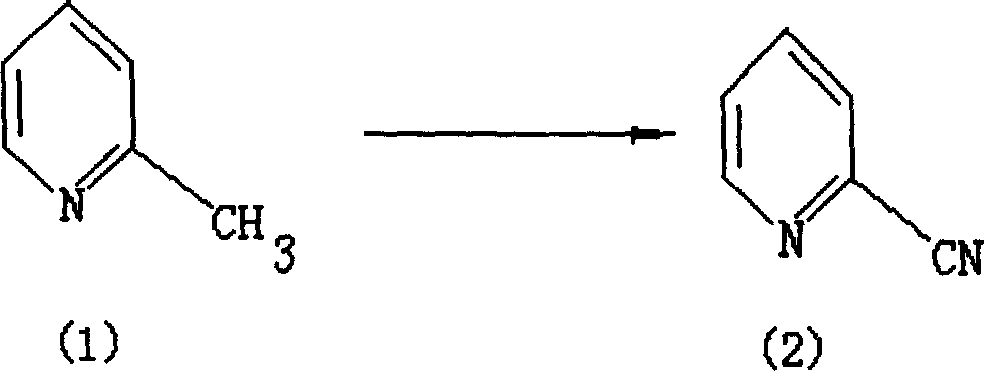
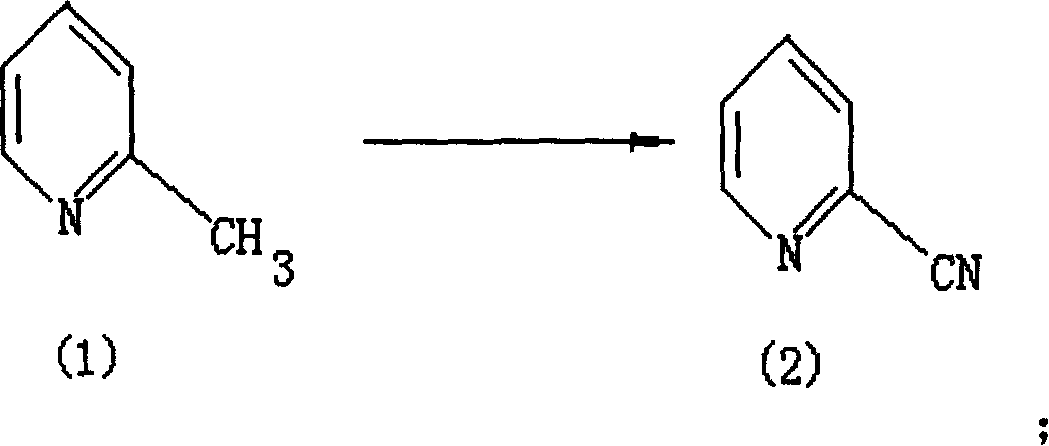Method for preparing compound in category of cyanopyridine
A technology for cyanopyridine and compounds, which is applied in the field of preparation of 2-cyanopyridine compounds, can solve the problems of long process route, difficult industrial implementation, and low reaction yield, and achieve the effect of short process route and convenient industrial implementation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Catalyst preparation:
[0037] (1) Put 50 grams of V 2 o 5 with 80 g Zr 2 o 2 Dissolve in 300ml of sulfuric acid aqueous solution with a weight concentration of 70%, add 20 grams of carrier inert alumina balls, soak for 2 hours, filter, collect the filter cake, and dry at 120°C for 3 hours;
[0038] (2) immerse the product of step (1) in 100 ml of Cr-containing sulfuric acid aqueous solution with a weight concentration of 25% for 3 hours, filter, collect the filter cake, and dry at 120° C. for 3 hours;
[0039] (3) The product of step (2) is immersed in 85ml containing Al 2 o 3 The weight concentration is 10% sulfuric acid aqueous solution for 5 hours, filter, collect filter cake, 120 ℃ dry 24 hours, obtain catalyst 1;
[0040] The general formula of the catalyst obtained is as follows:
[0041] (V a Zr b x c Y d o e ) f (Z) g
[0042] Wherein: a=11; b=15; c=5; d=3; e=7; f=95% by weight; g=5% by weight.
Embodiment 2
[0044] The preparation of catalyst is identical with embodiment 1, wherein:
[0045] X is Fe;, Y is Si;
[0046] The general formula of the catalyst obtained is as follows:
[0047] (V a Zr b x c Y d o e ) f (Z) g
[0048] Wherein: a=10; b=13; c=5; d=3; e=6; f=97% by weight; g=3% by weight.
[0049] Catalyst 2 is obtained.
Embodiment 3
[0051] The preparation of the catalyst was the same as in Example 1.
[0052] Carrier is desulfurization active carbon, in step (1), 110 dry 24 hours, wherein:
[0053] a=13; b=15; c=0; d=0; e=2; f=100% by weight; g=0% by weight to obtain catalyst 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com