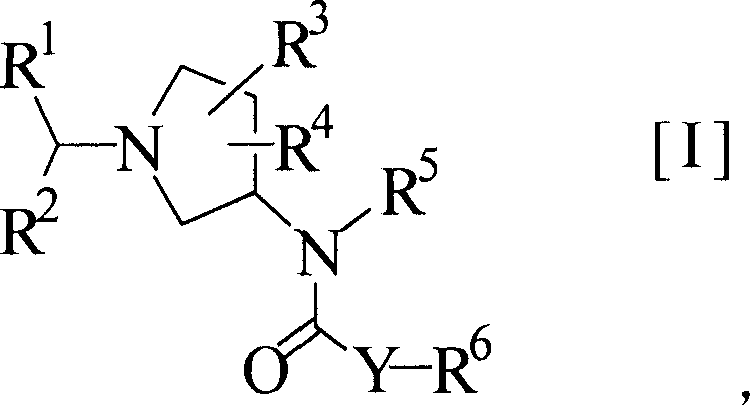
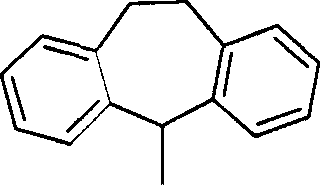
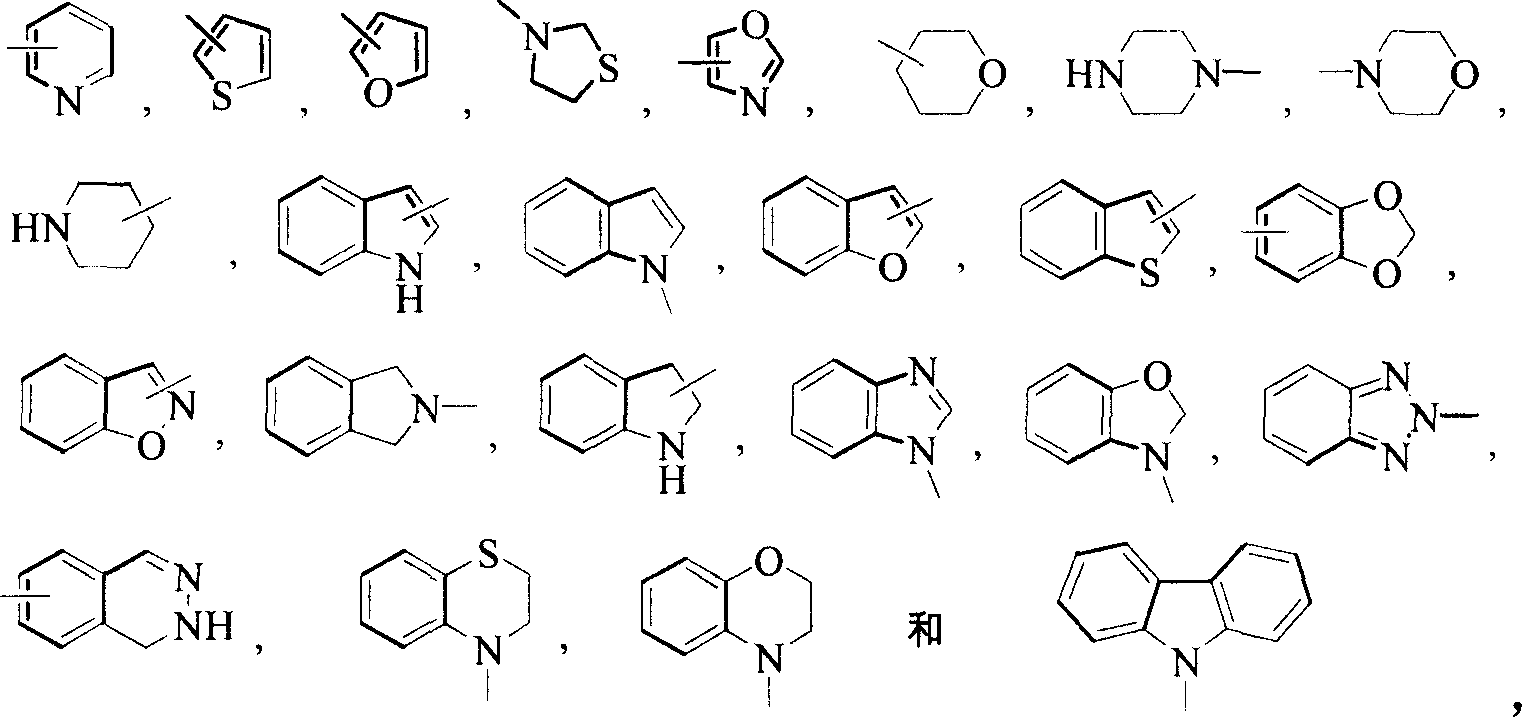
Pyrrolidine derivatives as CB1-receptor antagonists
A technology of alkyl and compounds, applied in the field of novel pyrrolidine compounds or their pharmaceutically acceptable salts, can solve the problem of not providing antagonistic activity of pyrrolidine compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0302] (3R)-1-[bis(4-chlorophenyl)methyl]-3-aminopyrrolidine (2.05 g, the compound prepared in Reference Example 11) and triethylamine (1.35 mL ) in dichloromethane (30 mL) was added dropwise 4-(trifluoromethoxy)benzoyl chloride (1.21 mL), and the mixture was stirred at room temperature for one hour. Water was added to the reaction mixture, and the mixture was extracted twice with chloroform. After filtering the organic layer through NH-silica gel (10 g of Chromatorex NH-silica gel; Fuji Silicia Chem.), the filtrate was evaporated under vacuum. The crude product was triturated in ethyl acetate / hexanes to give (3R)-1-[bis(4-chlorophenyl)methyl]-3-[[4-(trifluoromethoxy)benzoyl ]amino]pyrrolidine (2.70 g; yield: 83%) crystals.
[0303] MS(APCI) m / z: 508 / 510[M+H] + (MS(APCI): Atmospheric Pressure Chemical Ionization Mass Spectrometry)
Embodiment 2
[0305] (1) The product (2.01 g) obtained in Reference Example 8 was treated in the same manner as described in Reference Example 11-(2) and 6-(2), thereby obtaining (3,4-trans)-1-[di (4-Chlorophenyl)methyl]-3-hydroxy-4-aminopyrrolidine dihydrochloride (2.13 g; yield: 51%). MS(APCI) m / z: 337 / 339[M+H] +
[0306] (2) Add dropwise 4 - (trifluoromethoxy)benzoyl chloride (635 µL), and the mixture was stirred at the same temperature for 1 hour. The organic layer was separated and filtered through NH-silica gel beads (5 g Chromatorex NH-silica gel) and the filtrate was evaporated under vacuum. The crude product was triturated in ethyl acetate / hexanes to give (3,4-trans)-1-[bis(4-chlorophenyl)methyl]-3-hydroxy-4-[[4-( Trifluoromethoxy)benzoyl]amino]pyrrolidine (1.61 g; yield: 84%) crystals.
[0307] MS(APCI) m / z: 525 / 527[M+H] +
Embodiment 3
[0309] To a solution of the compound (26.9 mg) obtained in Reference Example 11 and 6-methylnicotinic acid (20.5 mg) in chloroform (1 mL) was sequentially added 0.5 M 1-hydroxybenzotriazole / dimethylformamide (0.4 mL ) and 0.5M 1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide hydrochloride / chloroform (0.4 mL), and the mixture was stirred at room temperature overnight. To the reaction mixture were added saturated aqueous sodium bicarbonate (1 mL), water (2 mL) and chloroform (2 mL), and the mixture was stirred for 15 minutes. The chloroform layer in the mixture was separated and evaporated under vacuum. The crude product was purified using HPLC (XTerra Prep MS C18 column; solvent: 10 mM ammonium carbonate / methanol=80:20→5:95), then dissolved in tert-butanol (1.5 mL), and lyophilized to obtain (3R)-1- [Bis(4-chlorophenyl)methyl]-3-[(6-methylnicotinoyl)amino]pyrrolidine powder (40.7 mg; yield: 92%). MS(ESI)m / z; 440 / 442[M+H] +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com