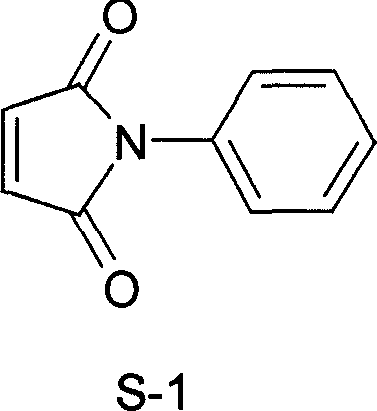
Process for preparing N-phenyl maleimide
A maleimide and synthetic method technology, applied in the direction of organic chemistry, can solve the problems of environmental pollution, high water solubility of DMF, and high cost, and achieve the effects of reducing environmental pollution, reducing production costs, and simplifying the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
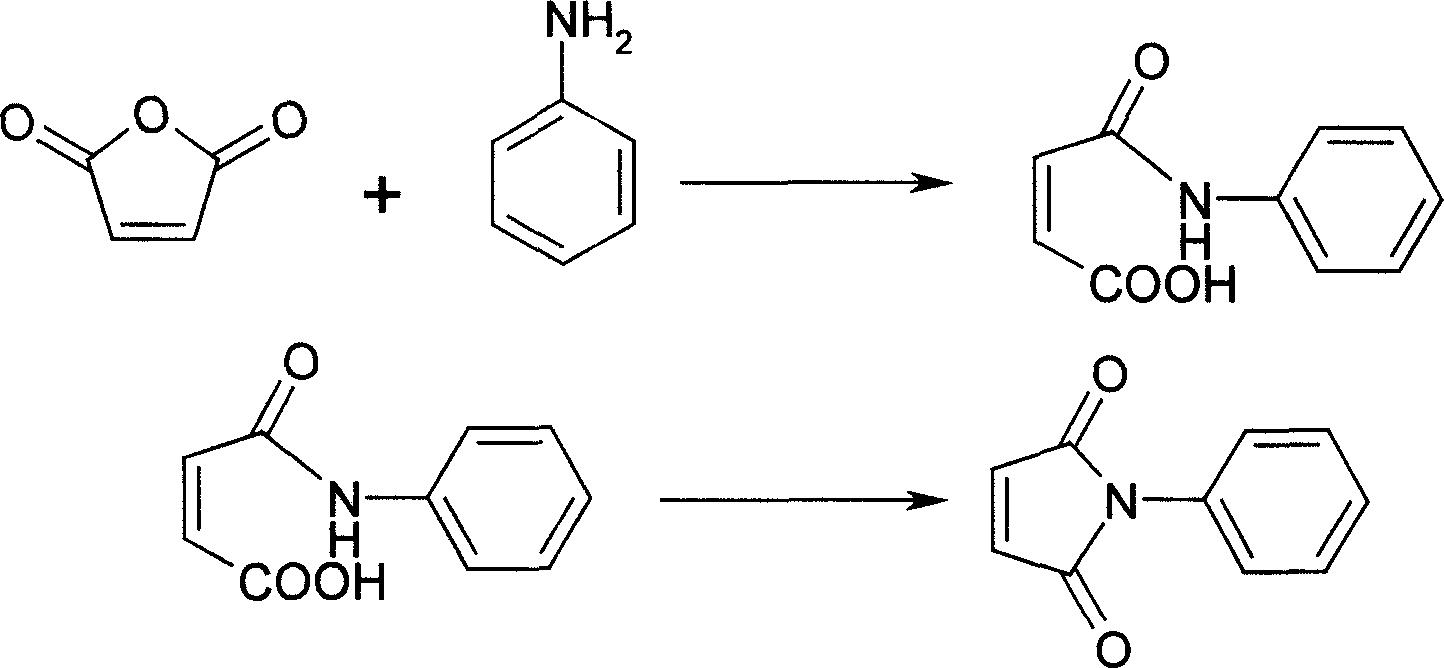
[0014] Embodiment 1: a kind of synthetic method of N-phenylmaleimide, take maleic anhydride and aniline as main starting raw material, make through following steps successively:
[0015] (1) Acylation, the preparation of N-phenyl maleamic acid:
[0016] In the flask equipped with stirring, dropping funnel and thermometer, add maleic anhydride (25.0g, 0.26mol), toluene (120mL), add aniline (23.0mL, 0.26mol) in the dropping funnel, heat, 60°C Aniline was added dropwise, and the dropwise addition was completed within 1 hour to obtain a white milky solid.
[0017] 2) Dehydration to form a ring, preparation of N-phenylmaleimide crude product:
[0018] Add p-toluenesulfonic acid (2g, 0.01mol), hydroquinone (1g, 0.01mol) and N-ethylpyrrolidone (0.8mL, 0.80g), heat, and start dehydration when the temperature reaches 90°C, and when it reaches 110°C, Steady water flow. After 8 hours of reaction, anhydrous was formed, and the reaction was completed, and a reddish-brown transparent sol...
Embodiment 2
[0021] Embodiment 2: a kind of synthetic method of N-phenylmaleimide, take maleic anhydride and aniline as main starting raw material, make through following steps successively:
[0022] (1) Acylation, the preparation of N-phenylmaleic acid:
[0023] In the flask equipped with stirring, dropping funnel and thermometer, add maleic anhydride (17.8g, 0.18mol), toluene (120mL), add aniline (11.5mL, 0.13mol) in the dropping funnel, heat, 40°C Aniline was added dropwise, and the dropwise addition was completed within 5 hours to obtain a white milky solid.
[0024] 2) dehydration, preparation of N-phenylmaleimide:
[0025] Add p-toluenesulfonic acid (1g, 0.005mol), hydroquinone (0.5g, 0.005mol) and N-ethylpyrrolidone (0.7mL), heat, dehydration starts at 90°C, and stabilizes at 110°C. After 2 hours of reaction, anhydrous was formed, and the reaction was completed, and a reddish-brown transparent solution was obtained. The toluene was removed on a rotary evaporator to obtain 30 g of...
Embodiment 3
[0028] Embodiment 3: a kind of synthetic method of N-phenylmaleimide, take maleic anhydride and aniline as main starting raw material, make through following steps successively:
[0029] (1) Acylation, the preparation of N-phenyl maleamic acid:
[0030] In the flask equipped with stirring, dropping funnel and thermometer, add maleic anhydride (30.0g, 0.31mol), xylene (190mL), add aniline (23.0mL, 0.26mol) in the dropping funnel, heat, 20 The aniline was added dropwise at ℃, and the dropwise addition was completed within 2 hours to obtain a white milky solid.
[0031] 2) dehydration, preparation of N-phenylmaleimide:
[0032] Add p-toluenesulfonic acid (2g, 0.01mol), hydroquinone (1g, 0.01mol) and N-ethylpyrrolidone (1.2mL), heat, dehydration starts at 90°C, and stabilizes at 138°C. After 4 hours of reaction, anhydrous was formed, and the reaction was completed, and a reddish-brown transparent solution was obtained. The toluene was removed on a rotary evaporator to obtain 53...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com