Roxithromycin injection and preparation process thereof
A technology of roxithromycin and roxithromycin aspartate is applied in the field of preparation of roxithromycin aspartate freeze-dried powder injection and solution injection, can solve problems such as incompatibility and waste, and achieves Effective bacteriostatic concentration, suitable pH value, good application prospect and significance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1: the preparation of roxithromycin aspartate for injection:
[0016] The prescription consists of: (calculated on the basis of 100 sticks)
[0017] Roxithromycin 15.0g
[0018] Aspartic Acid 2.4g
[0019] Mannitol 5.0g
[0020] Water for injection 200mL
[0021] Weigh the mannitol of the prescribed amount, add water for injection to make a solution with a concentration of about 15%, and set aside. Weigh the prescribed amount of roxithromycin and aspartic acid, put them in a 250mL beaker, add about 120mL of water for injection and the above-mentioned mannitol solution, stir at room temperature until completely dissolved, add 0.16g of activated carbon for needles, and Stir for 20 minutes, filter, wash the charcoal cake twice with the remaining water for injection, combine the original solution of the washing liquid, pass through a 0.22 μm microporous membrane filter for sterilization, pack aseptically according to the predetermined dose, and freeze-dry to ...
Embodiment 2
[0022] Embodiment 2: the preparation of roxithromycin aspartate injection:
[0023] The prescription consists of: (calculated on the basis of 100 sticks)
[0024] Roxithromycin 15.0g
[0025] Aspartic Acid 2.4g
[0026] Water for injection 200mL
[0027] Weigh the prescribed amount of roxithromycin and aspartic acid, place in a 250mL beaker, add about 160mL of water for injection, stir at room temperature until fully dissolved, add 0.16g of activated carbon for needles, stir at 40°C for 20 minutes, filter After that, the charcoal cake was washed twice with the remaining water for injection, and the stock solution of the lotion was combined and filtered through a 0.45 μm microporous membrane, divided into ampoules according to the predetermined dose, and sterilized by circulating steam at 100°C for 30 minutes.
Embodiment 3
[0028] Embodiment 3: the preparation of roxithromycin aspartate infusion:
[0029] The prescription consists of: (calculated on the basis of 100 sticks)
[0030] Roxithromycin 15.0g
[0031] Aspartic Acid 2.4g
[0033] Water for injection 10000mL
[0034] Weigh the prescribed amount of roxithromycin, aspartic acid and sodium chloride, put them in a 10000mL beaker, add about 8000mL of water for injection, stir at room temperature until completely dissolved, add 80.0g of activated carbon for needles, and stir at 40°C After 20 minutes, filter, wash the charcoal cake twice with the remaining water for injection, combine the original solution of the lotion and filter through a 0.45 μm microporous membrane, pack it into infusion bottles according to the predetermined dose, and sterilize it at 115°C for 30 minutes. .
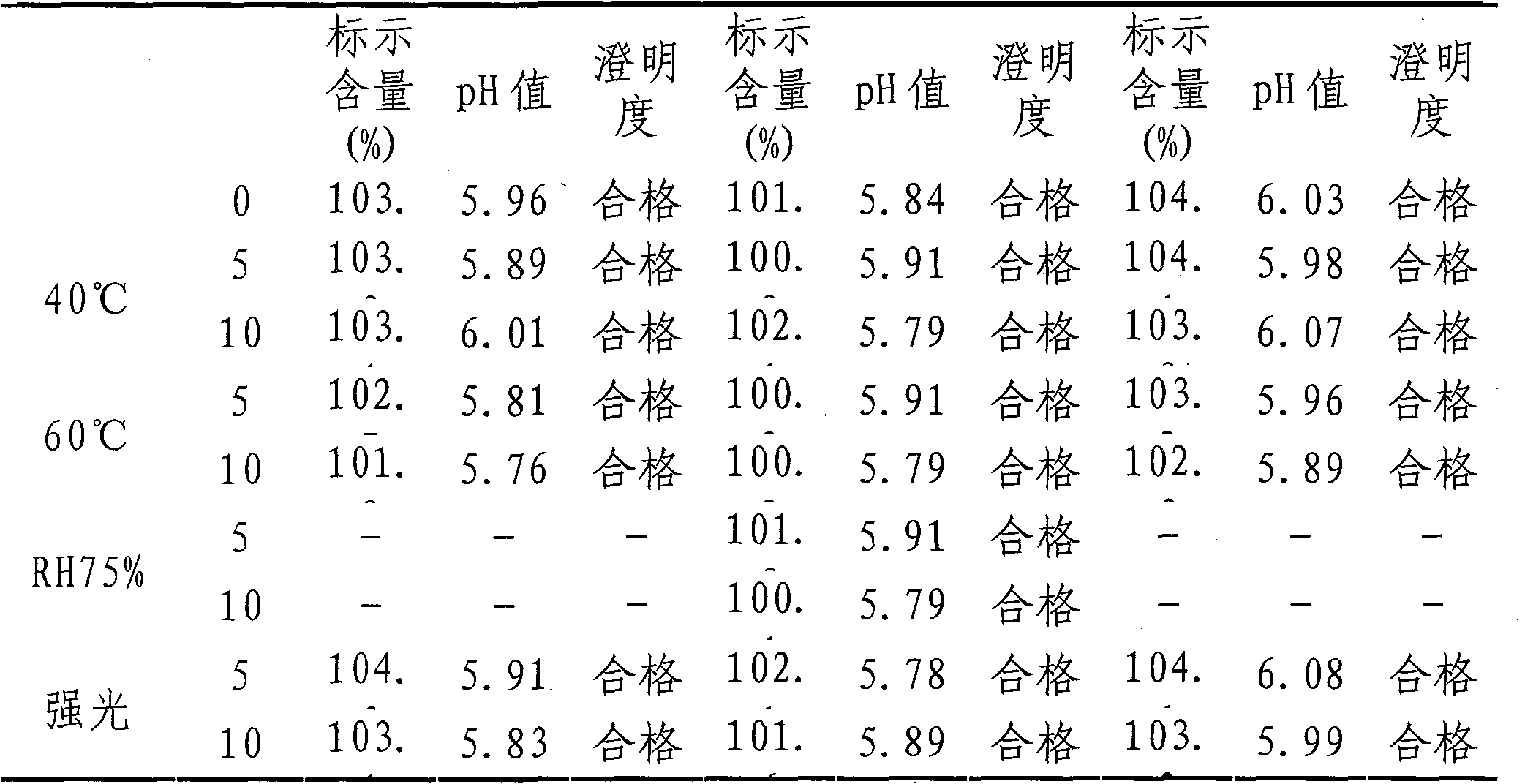
[0035] The following test data can illustrate the benefits of the present invention:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com