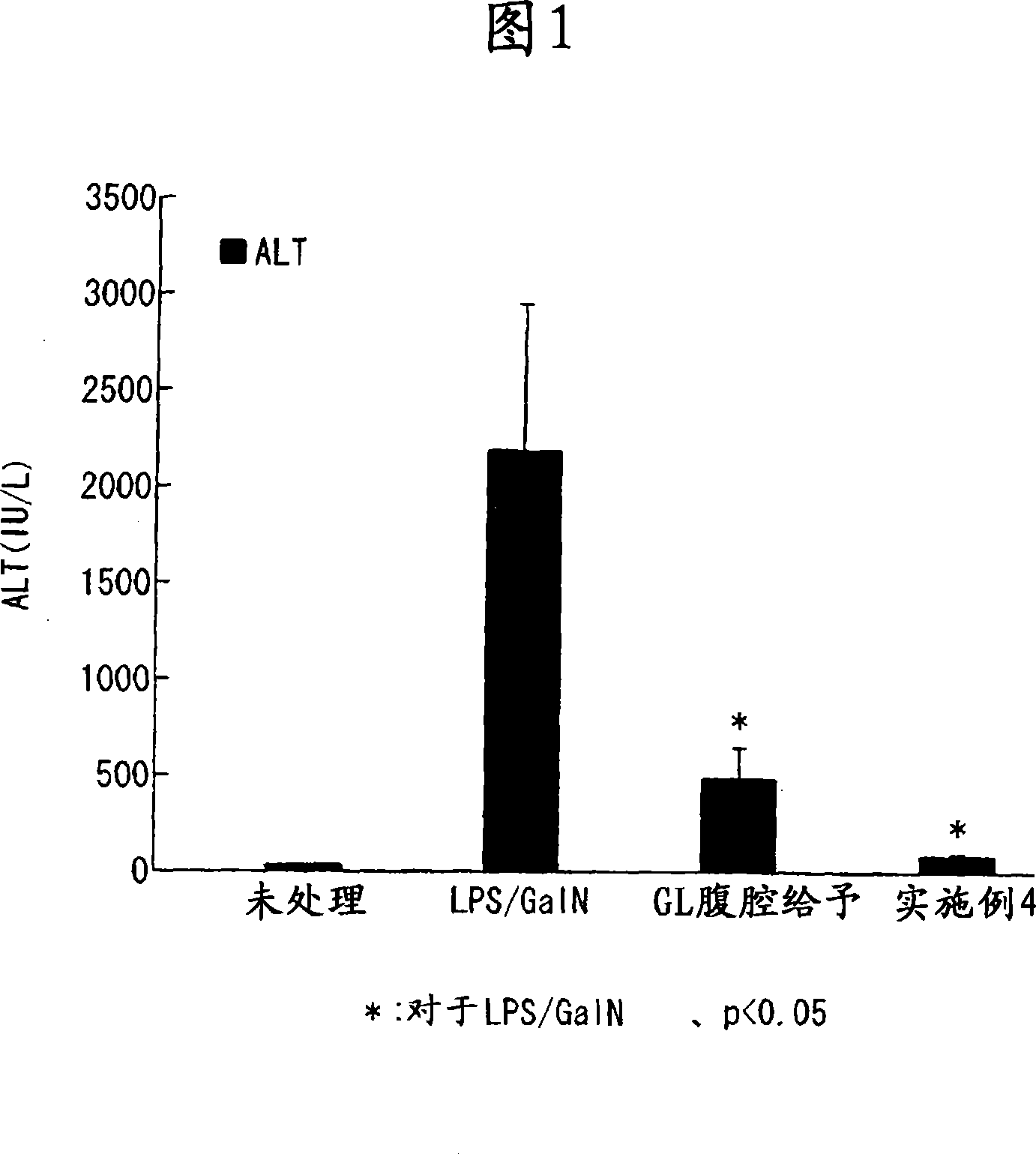
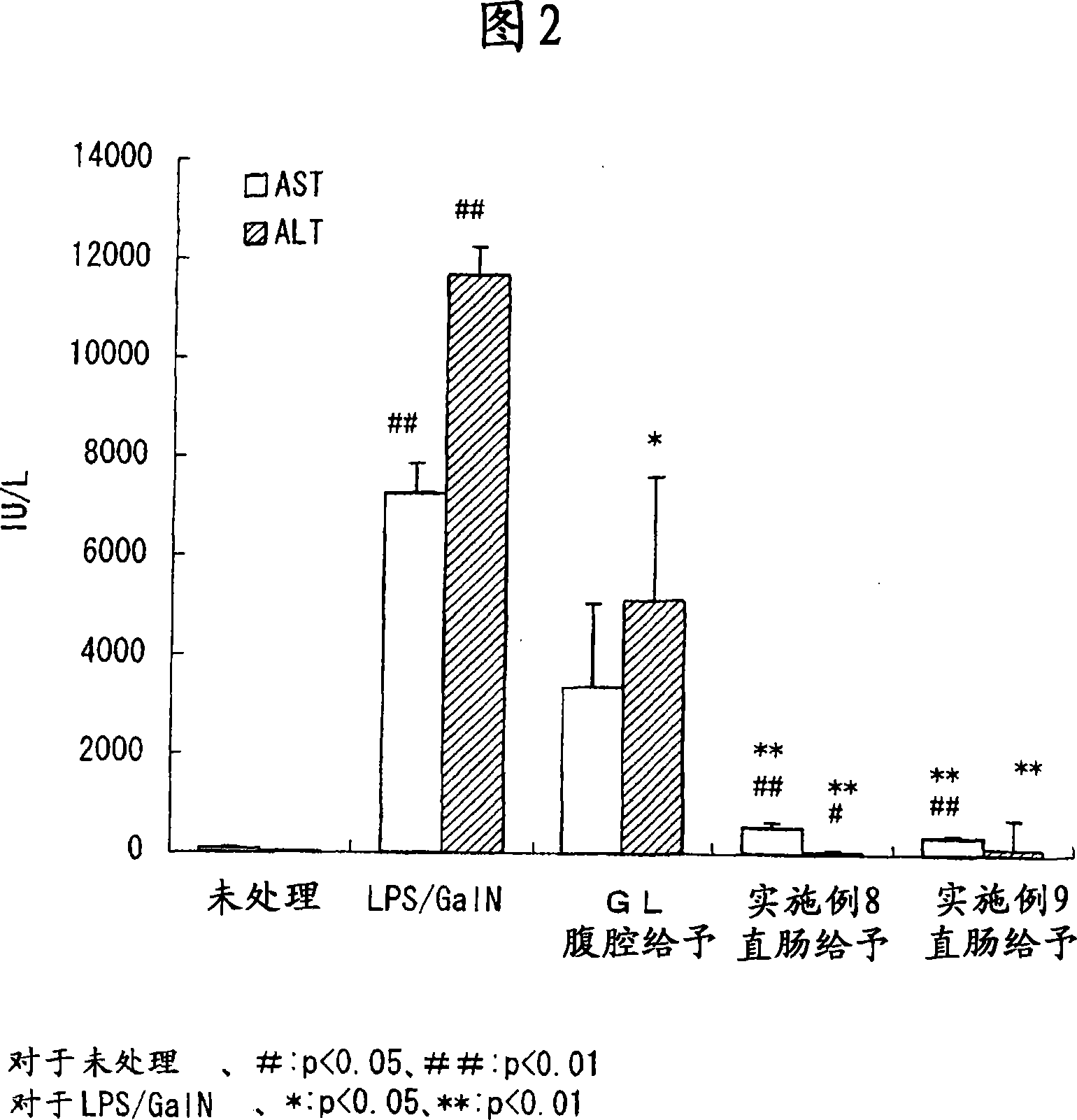
Glycyrrhizin-containing suppository compositions for rectal infusion
A glycyrrhizic, rectal technique
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~6 and comparative example 1
[0137] Mix monoammonium glycyrrhizinate and oily base agent according to the mixing amount (mass %) shown in Table 1, further add nonionic surfactant, stir evenly in a mortar, add water, carry out thorough mixing, and obtain the rectal injection containing GL Suppository compositions.
[0138] In addition, the abbreviations in Table 1 have the following meanings.
[0139] GL-NH 4 : Monoammonium glycyrrhizinate
[0140] J-1811: Sucrose fatty acid ester with HLB of 11 (manufactured by Mitsubishi Chemical Fu-zu Co., Ltd.)
[0141] S-24D: Polyglycerol fatty acid ester with HLB of 10 (manufactured by Mitsubishi Chemical Fu-zu Co., Ltd.)
[0142] L-7D: Polyglycerol fatty acid ester with HLB of 17 (manufactured by Mitsubishi Chemical Fu-zu Co., Ltd.)
[0143] PGMSV: Polyglycerol fatty acid ester with HLB of 14 (manufactured by Nisshin Oirio Co., Ltd.)
[0144] ミブリオ-ル: ミブリオ-ル812 (medium-chain fatty acid triglycerides with a fatty acid composition of 50-65% caprylic acid and 30-45...
Embodiment 14~27
[0228] According to the composition and mixing amount (mass %) shown in Table 9~11, utilize homogenizer, evenly suspend monoammonium glycyrrhizinate (GL-NH 4 ), an oily base, a nonionic surfactant, purified water or an aqueous alkali solution, and fully stirred to prepare a rectal injection suppository composition containing GL. In addition, in Example 25, gerline (Benzalkonium salt) was further added to prepare a composition.
[0229] In Examples 19 to 27 using the above-mentioned aqueous alkali solution, the ratio (mass %) of the water component and the alkali component in the composition is shown in parentheses.
[0230] In addition, the abbreviations in Tables 9 to 11 have the following meanings.
[0231] SS: Sucrose fatty acid ester with HLB of 19 (manufactured by Daiichi Kogyo Pharmaceutical Co., Ltd.)
[0232] F-160: Sucrose fatty acid ester with HLB of 15 (manufactured by Daiichi Kogyo Pharmaceutical Co., Ltd.)
[0233] J-1811: HLB is 11 sucrose fatty acid ester (ma...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com