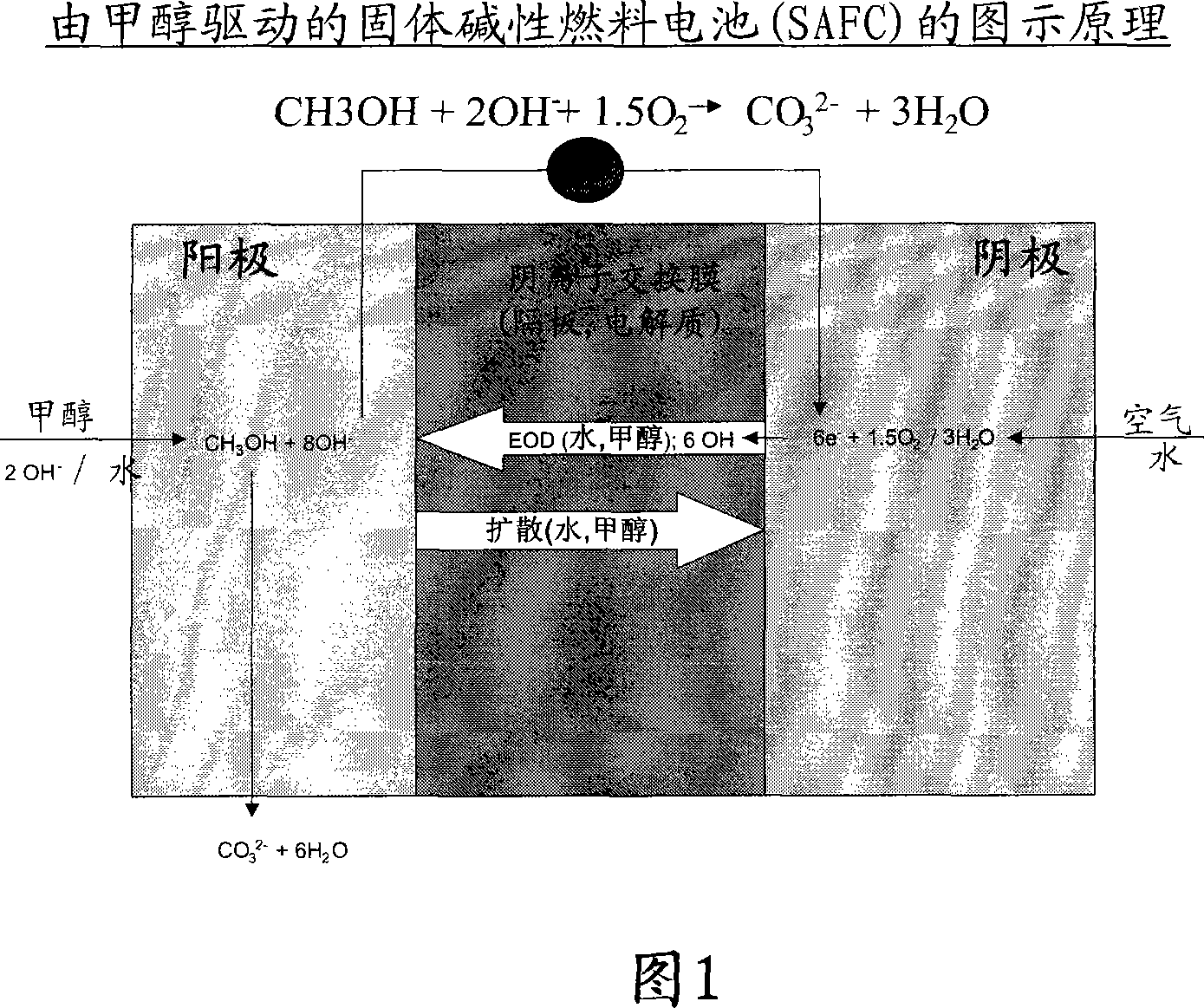
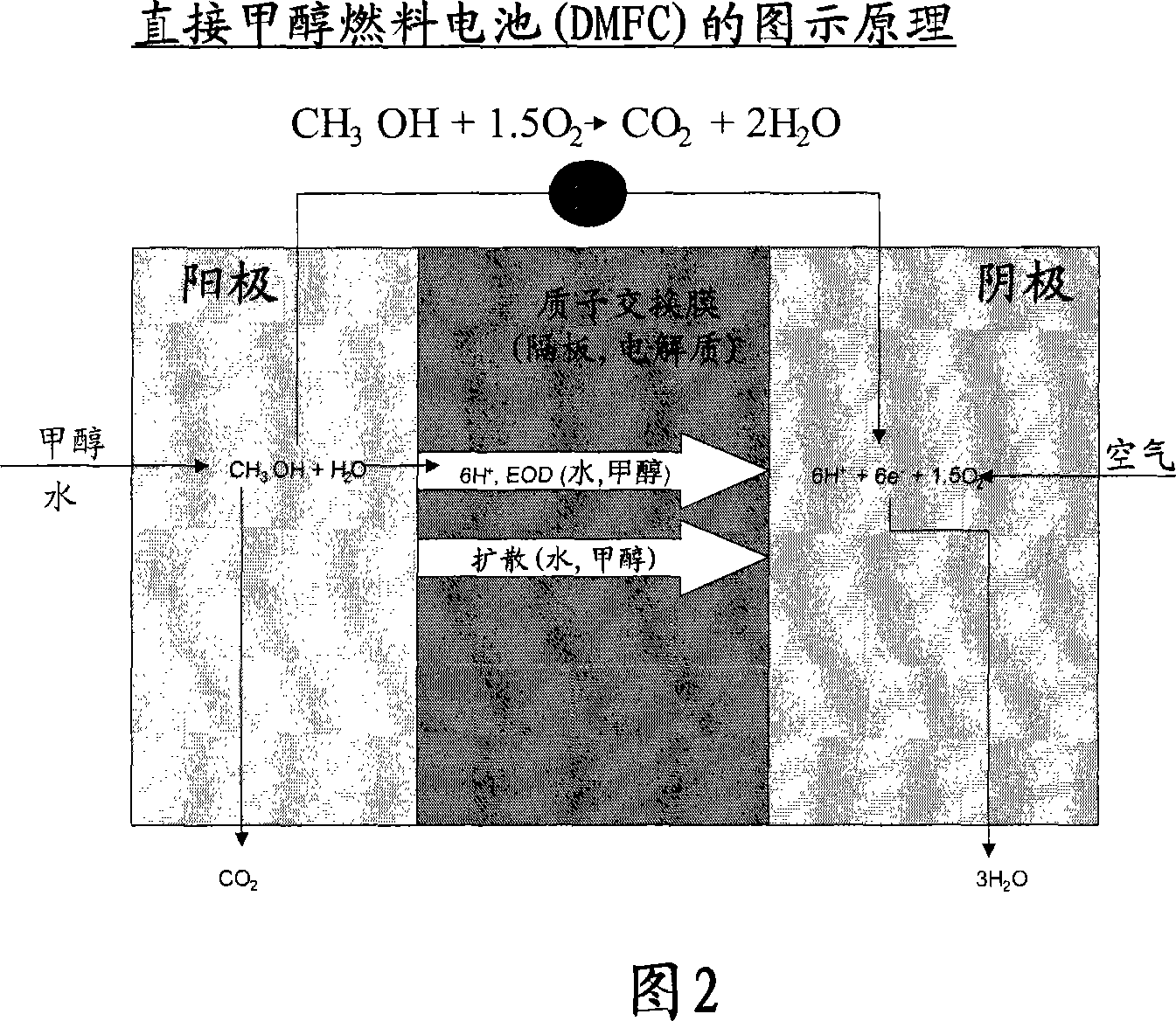
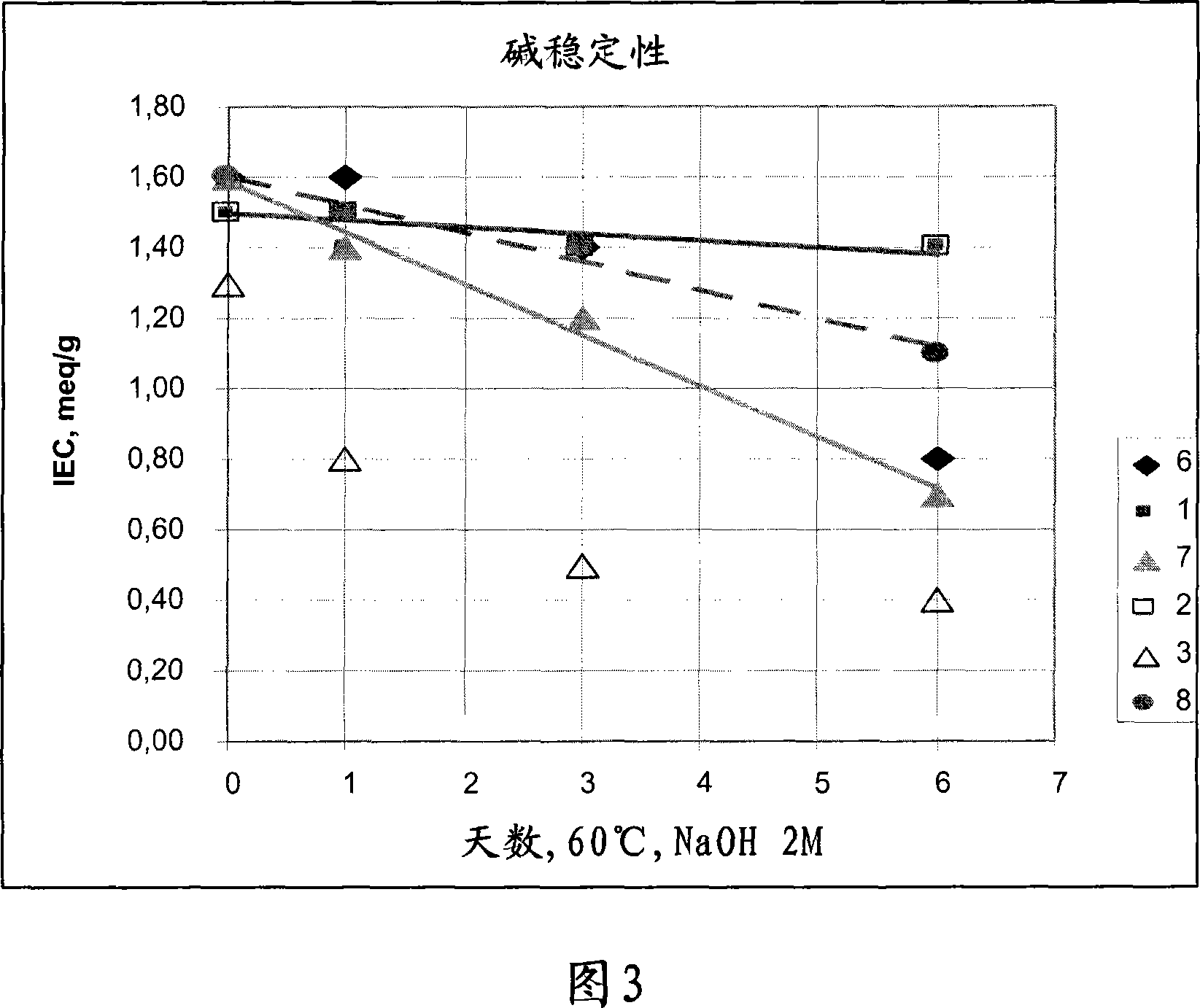
Solid alkaline fuel cell comprising ion exchange membrane
An anion-exchange membrane and fuel cell technology, applied in the field of ion-exchange membranes, can solve problems such as unsatisfactory performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0171] Example 1 : N,N,2,2-tetramethyl-1,3-propanediamine-ETFE-g-St carrier
[0172] 50 μm ETFE membranes were modified by radiation grafting using a mixture of styrene / divinylbenzene (3% vol) up to a degree of grafting of 50%w.
[0173] The grafted copolymer ETFE-g-St (50%w) was then treated as follows:
[0174] 1. Chlorosulfonation was performed by immersing the membrane in a large excess of 5% vol chlorosulfonic acid (CAS) in 1,2-dichloroethane. The reaction was carried out at 60°C for 4 hours.
[0175] 2. Extract unreacted CSA with diethyl ether at room temperature.
[0176] 3. Amination of chlorosulfonyl functional groups by contacting the membrane with a large excess of 5% vol N,N,2,2-tetramethyl-1,3-propanediamine in acetonitrile, the reaction was carried out at 60 °C for 16 Hour.
[0177] 4. Wash the aminated membrane sequentially with (1) 1 N NaOH solution, (2) ethanol at 60°C.
[0178] 5. Final quaternization by exposing the membrane to a large excess of 1 M so...
Embodiment 2
[0180] Example 2: N,N,2,2-tetramethyl-1,3-propanediamine-ETFE-g-St carrier
[0181] A 30 μm ETFE membrane was modified by radiation grafting using a mixture of styrene / divinylbenzene (3% vol) up to a degree of grafting of 42%w.
[0182] The grafted copolymer ETFE-g-St (42%w) was then treated as follows:
[0183] 1. Chlorosulfonation was performed by immersing the membrane in a large excess of 5% vol chlorosulfonic acid (CAS) in 1,2-dichloroethane. The reaction was carried out at 60°C for 2 hours.
[0184] 2. Extract unreacted CSA with diethyl ether at room temperature.
[0185] 3. Amination of chlorosulfonyl functional groups by contacting the membrane with a large excess of 5% vol of N,N,2,2-tetramethyl-1,3-propanediamine in acetonitrile, the reaction was carried out at 60 °C 6 hours.
[0186] 4. Wash the aminated membrane sequentially with (1) 1 N NaOH solution, (2) ethanol at 60°C.
[0187] 5. Final quaternization by exposing the membrane to a large excess of 1 M sol...
Embodiment 3
[0189] Example 3 : N-methylpiperazine-ETFE-g-St carrier
[0190] A 30 μm ETFE membrane was modified by radiation grafting using a mixture of styrene / divinylbenzene (3% vol) up to a degree of grafting of 42%w.
[0191] The grafted copolymer ETFE-g-St (42%w) was then treated as follows:
[0192] 1. Chlorosulfonation was performed by immersing the membrane in a large excess of 5% vol chlorosulfonic acid (CAS) in 1,2-dichloroethane. The reaction was carried out at 60°C for 2 hours.
[0193] 2. Extract unreacted CSA with diethyl ether at room temperature.
[0194] 3. Amination of the chlorosulfonyl functional group by contacting the membrane with a large excess of 5% vol N-methylpiperazine in acetonitrile, the reaction was carried out at 60°C for 16 hours.
[0195] 4. Wash the aminated membrane sequentially with (1) 1 N NaOH solution, (2) ethanol at 60°C.
[0196] 5. Final quaternization by exposing the membrane to a large excess of 1 M solution of methyl chloride in a mixtur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com