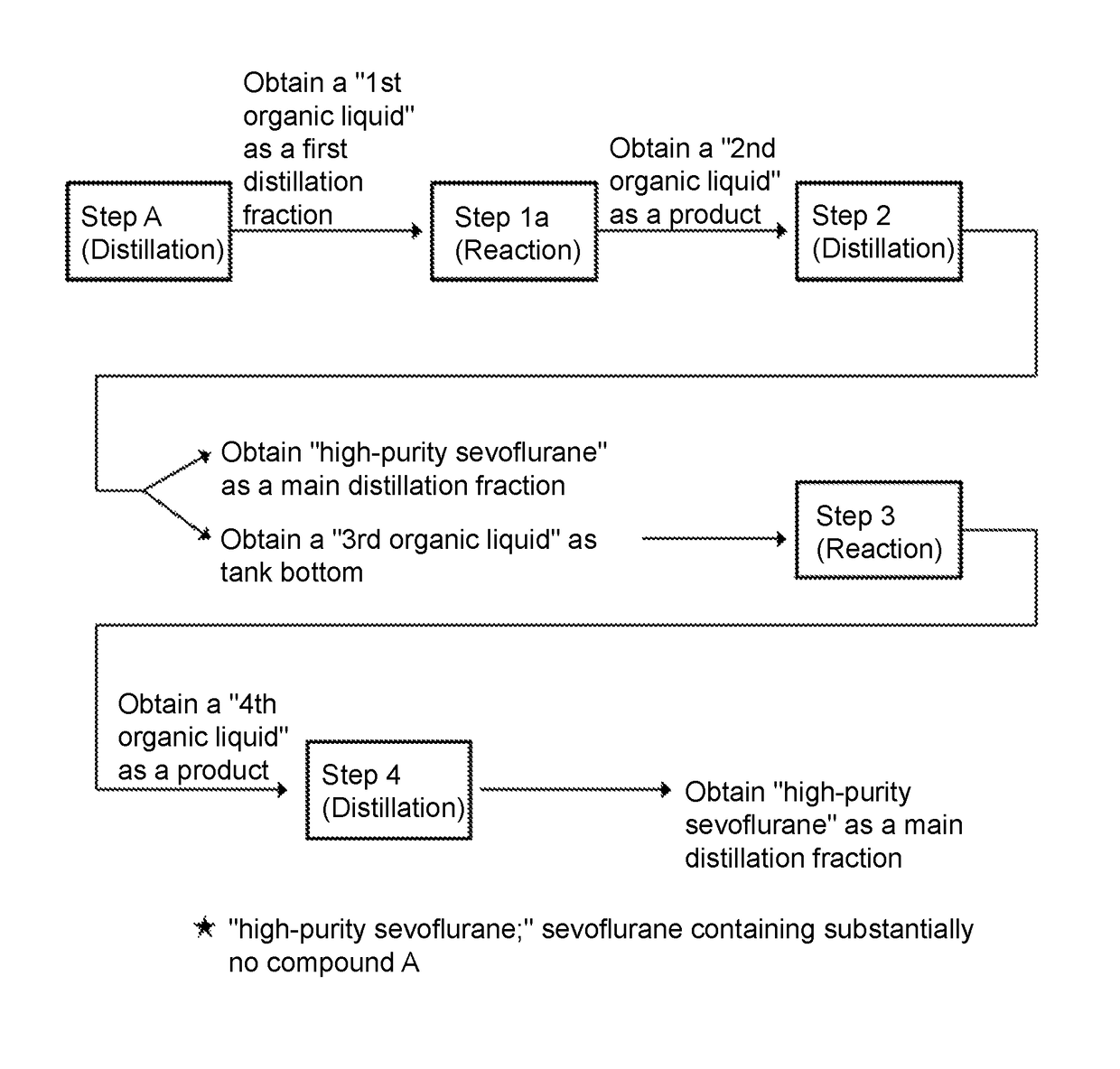
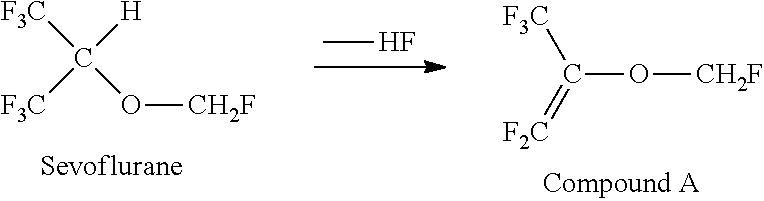
Method for producing sevoflurane
a technology of sevoflurane and sevoflurane, which is applied in the field of methods, can solve the problems of difficult separation of product and difficult separation of compound a from sevoflurane, and achieve the effects of effective use, effective use and convenient separation from sevofluran
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
(Step 1)
[0153]A compound A (purity: 99% or more) (5 g), HF (20 g), water (100 g), and 1,2-dichloroethane (DCE) serving as an internal standard substance (15 g) were mixed, followed by stirring in an air-tight polytetrafluoroethylene resin container for 5 hours at 20° C. to 25° C., thereby conducting a reaction. During the reaction, the peak area ratio of [compound A] / [DCE] was determined by FID gas chromatograph analysis at 1-hour intervals. Note that a sample liquid was brought into contact with NaF for dehydrofluorination and then analyzed by gas chromatography (the retention time for the compound A was around 5.2 minutes and the retention time for DCE was around 16.5 minutes under the gas chromatography conditions).
[0154]As a result, although the peak area ratio of [compound A] / [DCE] was 0.57 immediately before the start of the reaction, it became 0.48, 0.38, 0.31, 0.25, and 0.19 in 1, 2, 3, 4, and 5 hours, respectively, after the start of the reaction. In other words, after the ...
example 2
(Step A)
[0156]The standard substance of the compound A (used in Example 1) was used so that “sevoflurane containing compound A at 100 ppm” was prepared. The obtained sevoflurane (1000 g) was introduced into a glass distillation tank. In addition, a 1% sodium hydrogen phosphate aqueous solution (70 g) was added thereto, followed by ordinary pressure distillation using a distillation column with 10 theoretical stages at a reflux ratio of 5 to 20.
[0157]The distillate was analyzed by FID gas chromatography and collected as a “first distillation fraction” while the compound A was detectable at 1 ppm or more. Then, when the compound A was detected at a level of less than 1 ppm, the distillate was collected as a “main distillation fraction.”
[0158]As a result, the recovered amount of the “first distillation fraction” was 267 g, in which the content of compound A was 341 ppm. Meanwhile, the recovered amount of the “main distillation fraction” was 720 g, in which no compound A was detected (l...
example 3
(Step 1b)
[0170]In Example 3 and the subsequent examples, a reaction corresponding to step 1b was conducted at a small scale (one-tenth scale of Example 2) as a model experiment similar to step 1a of Example 2 (using a small sealable stainless-steel reactor).
[0171]At first, the standard substance of compound A and sevoflurane were mixed so that “sevoflurane containing compound A at 340 ppm” was prepared. The sevoflurane was designated as a reaction material in Example 3 and the subsequent examples.
[0172]An “HF aqueous solution (prepared by dissolving 1.0 g of anhydrous HF in 5.0 g of water)” was added to 24 g of the “sevoflurane containing compound A at 340 ppm.” Further, 1.0 g of HFIP was added and an autoclave was closed. Stirring was initiated using a magnetic stirrer and the interior temperature was maintained at 20° C. to 25° C.
[0173]After the elapse of 5 hours, FID gas chromatography was conducted for determination. The conversion rate of compound A was estimated as 73%. As HFI...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com