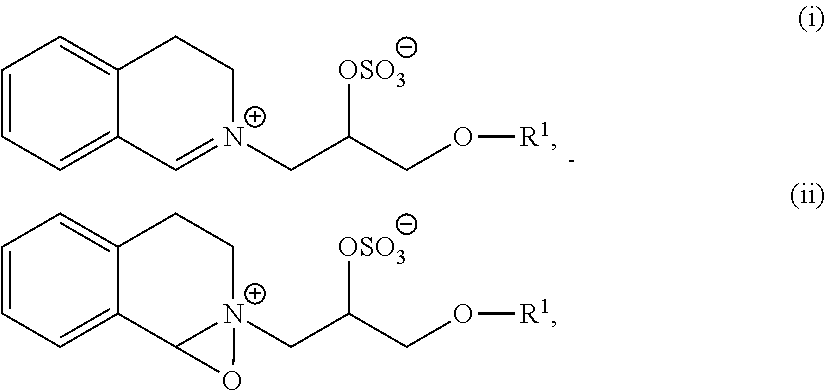
Subtilase variants with enhanced storage stability
a subtilase and storage stability technology, applied in the field of new products, can solve the problems of loss of wash performance during the wash cycle, many stains are still difficult to completely remove under conventional washing conditions, etc., and achieve the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
on and Expression of Variants
[1036]The following summarizes the mutation and introduction of an expression cassette into Bacillus subtilis. All DNA manipulations were done by PCR (e.g. Sambrook et al.; Molecular Cloning; Cold Spring Harbor Laboratory Press) and can be repeated by everybody skilled in the art.
[1037]Recombinant B. subtilis constructs encoding subtilase variants were used to inoculate shakeflasks containing a rich media (e.g. 100 g / L Sucrose (Danisco cat. no. 109-0429), 40 g / L crust soy (soy bean flour), 10 g / L Na2HPO4.12H2O (Merck cat. no. 6579), 0.1 ml / L replace-Dowfax63N10 (Dow). Cultivation typically takes 4 days at 30° C. shaking with 220 rpm.
example 2
ion of Variants
[1038]The culture broth was centrifuged (26000×g, 20 min) and the supernatant was carefully decanted from the precipitate. The supernatant was filtered through a Nalgene 0.2 μm filtration unit in order to remove the rest of the Bacillus host cells. pH in the 0.2 μm filtrate was adjusted to pH 8 with 3M Tris base and the pH adjusted filtrate was applied to a MEP Hypercel column (from Pall corporation) equilibrated in 20 mM Tris / HCl, 1 mM CaCl2, pH 8.0. After washing the column with the equilibration buffer, the column was step-eluted with 20 mM CH3COOH / NaOH, 1 mM CaCl2, pH 4.5. Fractions from the column were analysed for protease activity (using the Suc-AAPF-pNA assay at pH 9) and peak-fractions were pooled. The pH of the pool from the MEP Hypercel column was adjusted to pH 6 with 20% (v / v) CH3COOH or 3M Tris base and the pH adjusted pool was diluted with deionized water to the same conductivity as 20 mM MES / NaOH, 2 mM CaCl2, pH 6.0. The diluted pool was applied to a S...
example 3
ed Storage Stability Assay
[1039]Storage stability of protease variants in liquid detergent was evaluated using an accelerated assay with incubation at elevated temperatures for up to 24 hours.
[1040]All purified protease samples were diluted to concentrations of 0.2 and 0.1 mg / ml based on absorbance at 280 nm and theoretical extinction coefficient using 0.01% Triton X-100. For each variant 2 wells with high protease concentration and 2 wells with low concentration were included. As reference SEQ ID NO 3 was included on each microtiter plate. 30 μl protease sample was mixed with 270 μl detergent (Model 0 or CNS EDTA pH9) in the well of a microtiter plate (Nunc U96 PP 0.5 ml) using a magnetic bar (on Zephyr pipetting station (Caliper LifeSciences) for 30 min). 20 μl of this mixture was then transferred to another microtiter plate (Nunc U96 PP 0.5 ml with added magnetic bars) and mixed with 150 μl 100 mM Tris pH 8.6 (at least 5 min on Zephyr). 30 μl of this dilution was transferred to a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com