PAR2 mimetic peptides and uses thereof
a technology of mimetic peptides and par2, which is applied in the field of medical pharmacology, can solve the problems of difficult applicability, lack of suitable pharmacological tools, and hinder the full exploration of the role of par2 in disease conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
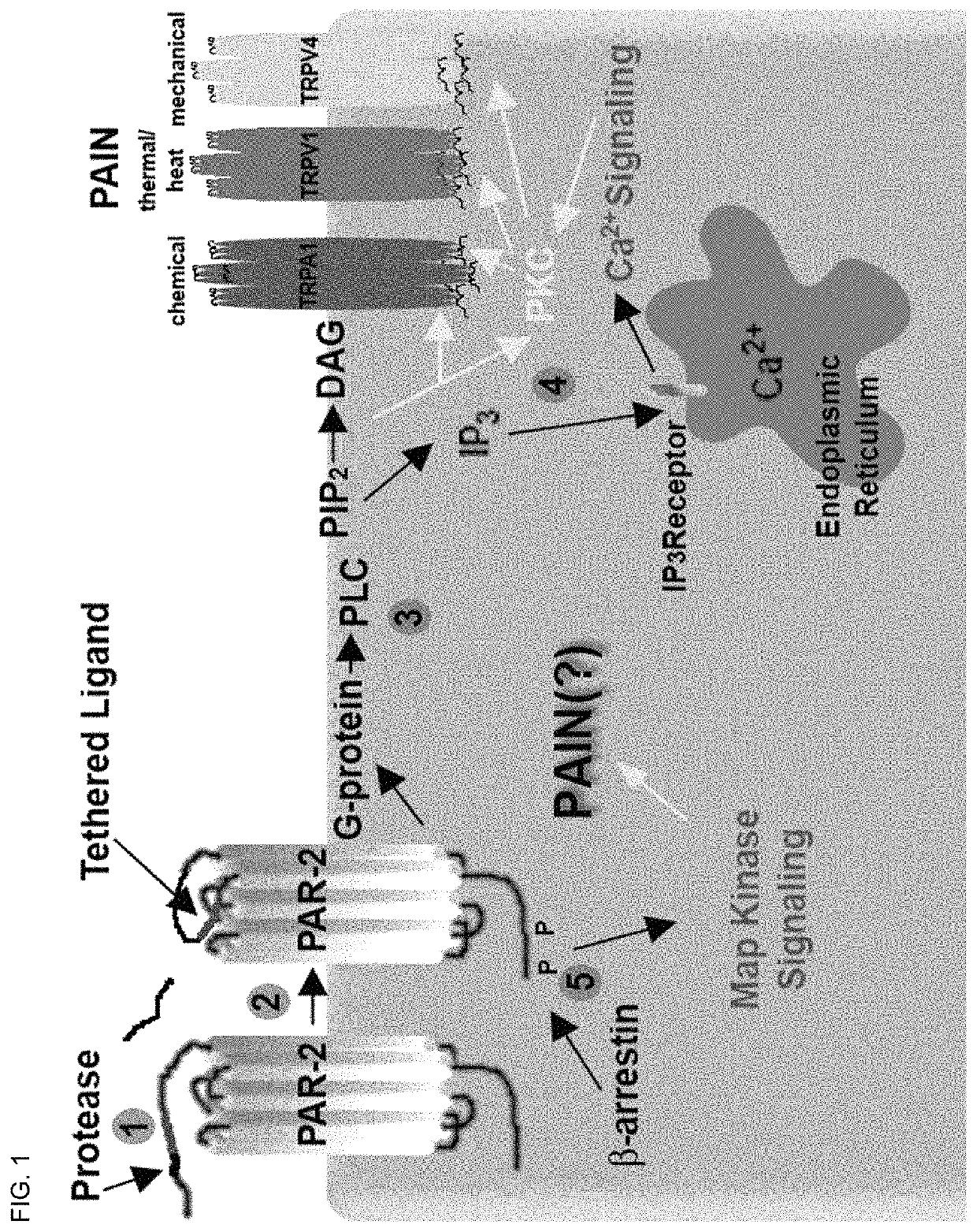
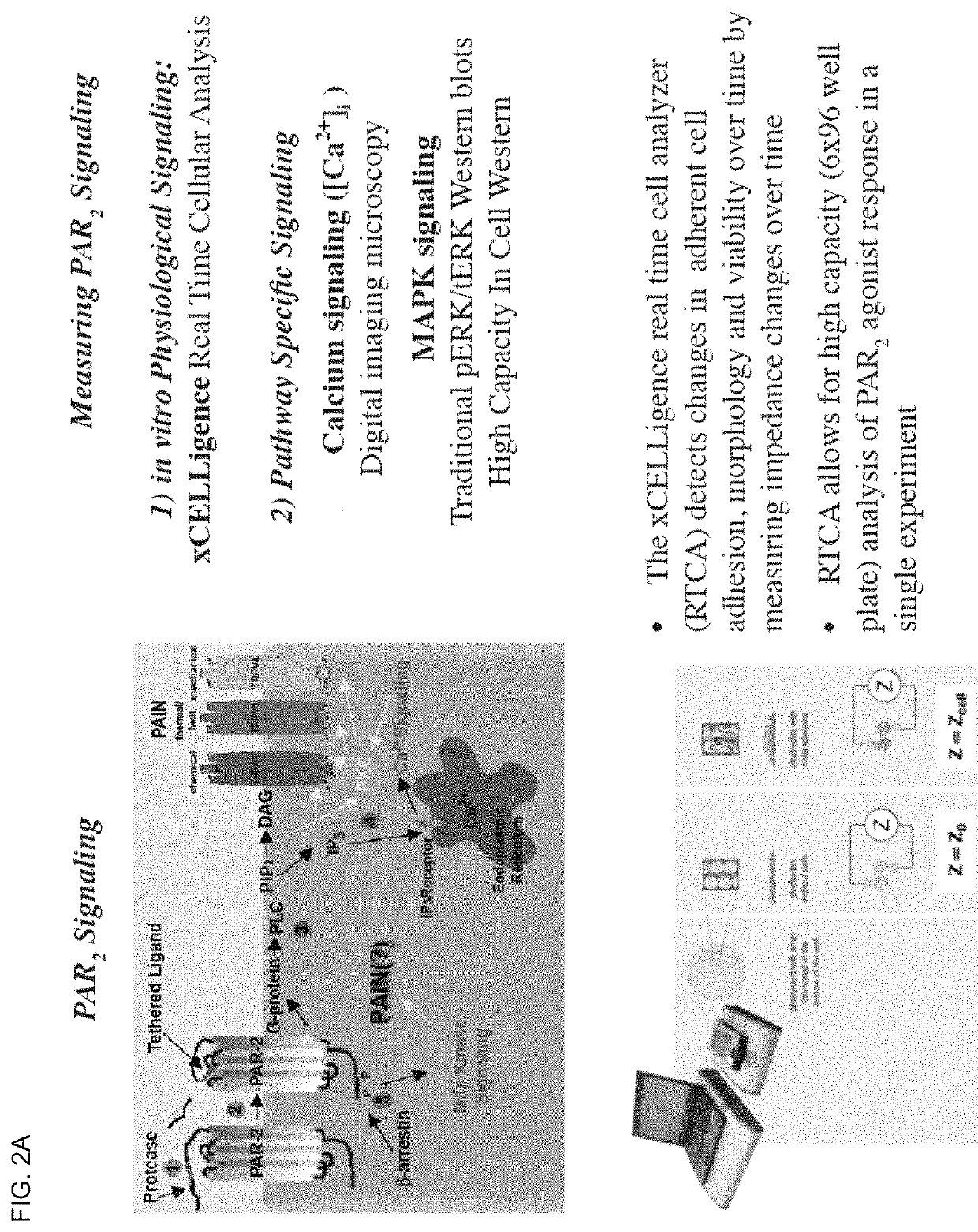
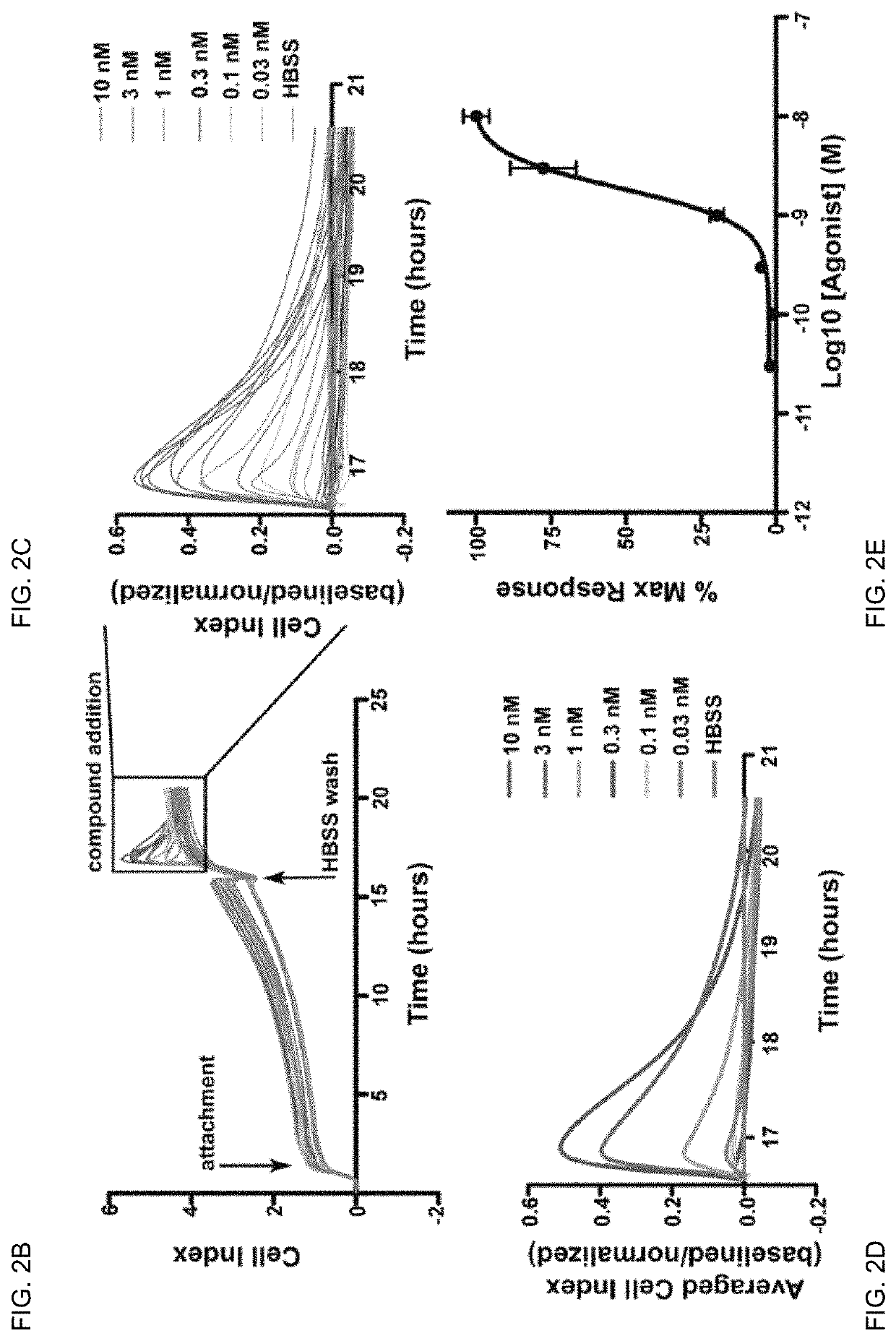
[0134]Protease-activated receptor-2 (PAR2) belongs to a four-member family of G-Protein coupled receptors (GPCRs) that contain internal ligands exposed following exogenous or endogenous protease cleavage of the extracellular amino terminus. PAR2 is associated with a variety of inflammatory conditions, including asthma and pain. The contributions of PAR2 signalling to disease has been hindered by the lack of potent, efficacious antagonists, and their potential for biased-ligand signalling. It was recently demonstrated that lipid tethering of known PAR2 peptidomimetic agonists based on the primary trypsin cleavage sequence (SLIGRL (SEQ ID NO: 19)) increased their potency >200 fold.
[0135]Here, lipid tethering (hexadecyl (Hdc) group with polyethylene glycol (PEG) spacers) and heterocycle (2-aminothiazoyl; 2-at) substitution of hexapeptide sequence derived from the primary cleavage site of kallikreins 4 / 16 (SSKGRS (SEQ ID NO:9)) was used to elucidate novel PAR2 antagonists. Compound 562 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| acid | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com