Hemoglobin-haptoglobin complexes
a technology of haptoglobin and complexes, which is applied in the field of haptoglobin complexes, can solve problems such as difficulty in identifying and treating metastases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
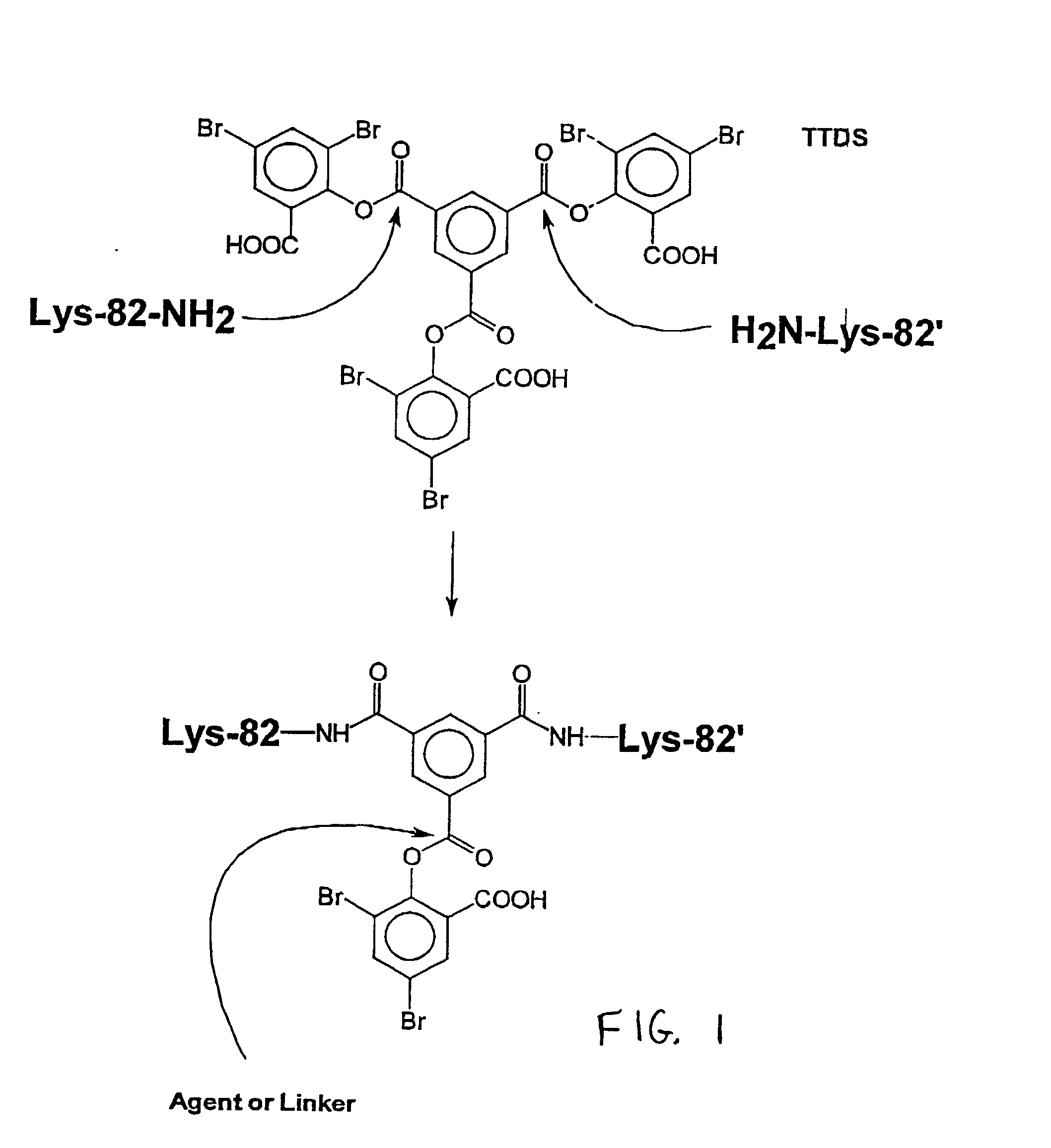
Conjugation of TTDS Cross-Linked Hemoglobin (THb) to Poly(L-lysine)
[0058] Poly(L-lysine) conjugates of TTDS cross-linked hemoglobin (THb-K.sub.n) were synthesized by adding poly(L-lysine) to THb-DBS (TTDS cross-linked hemoglobin with one unhydrolyzed 3,5-dibromosalicylate functionality) at 1:1 molar ratio to promote formation of conjugates in which only one molecule of hemoglobin is attached to a single poly(L-lysine) chain. The poly(L-lysine) used in this experiment is a linear polymer with an amide linkage between the carboxyl group and the .alpha.-amino group of lysine. Polymers with an average molecular weight of 4 kDa (K.sub.4kDa), 7.5 kDa (K.sub.7.5kDa), 26 kDa (K.sub.26kDa) and 37 kDa (K.sub.37kDa) were conjugated to THb.
[0059] TTDS (13.9 mg) in ethanol (100 .mu.L) was added to deoxyhemoglobin (5 mL, 8.5 g / dL) in 50 mM borate pH 9.0. The reaction mixture was stirred at 30.degree. C. under nitrogen for 45 min. The hemoglobin was then charged with CO (the solution was kept on i...
example 2
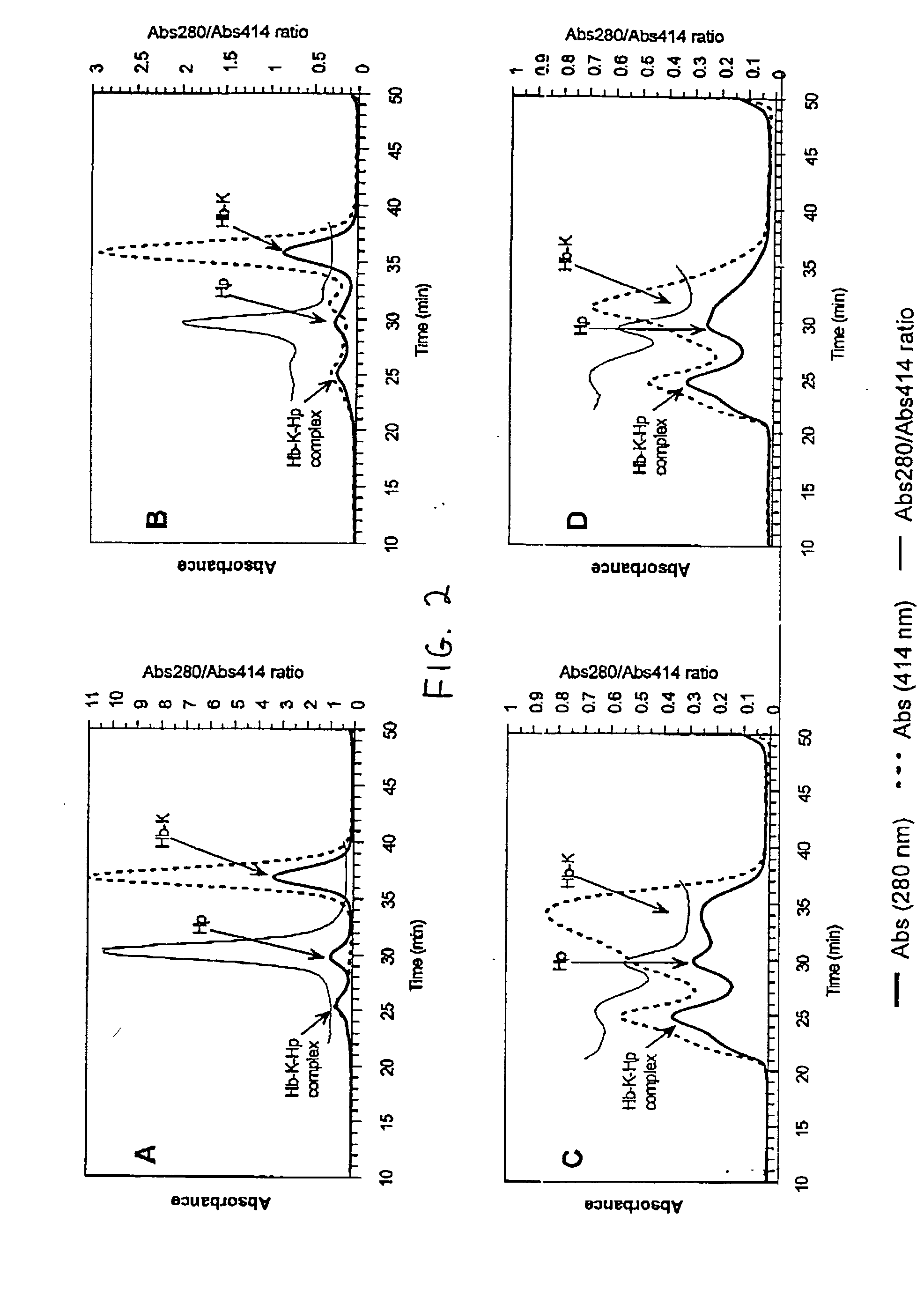
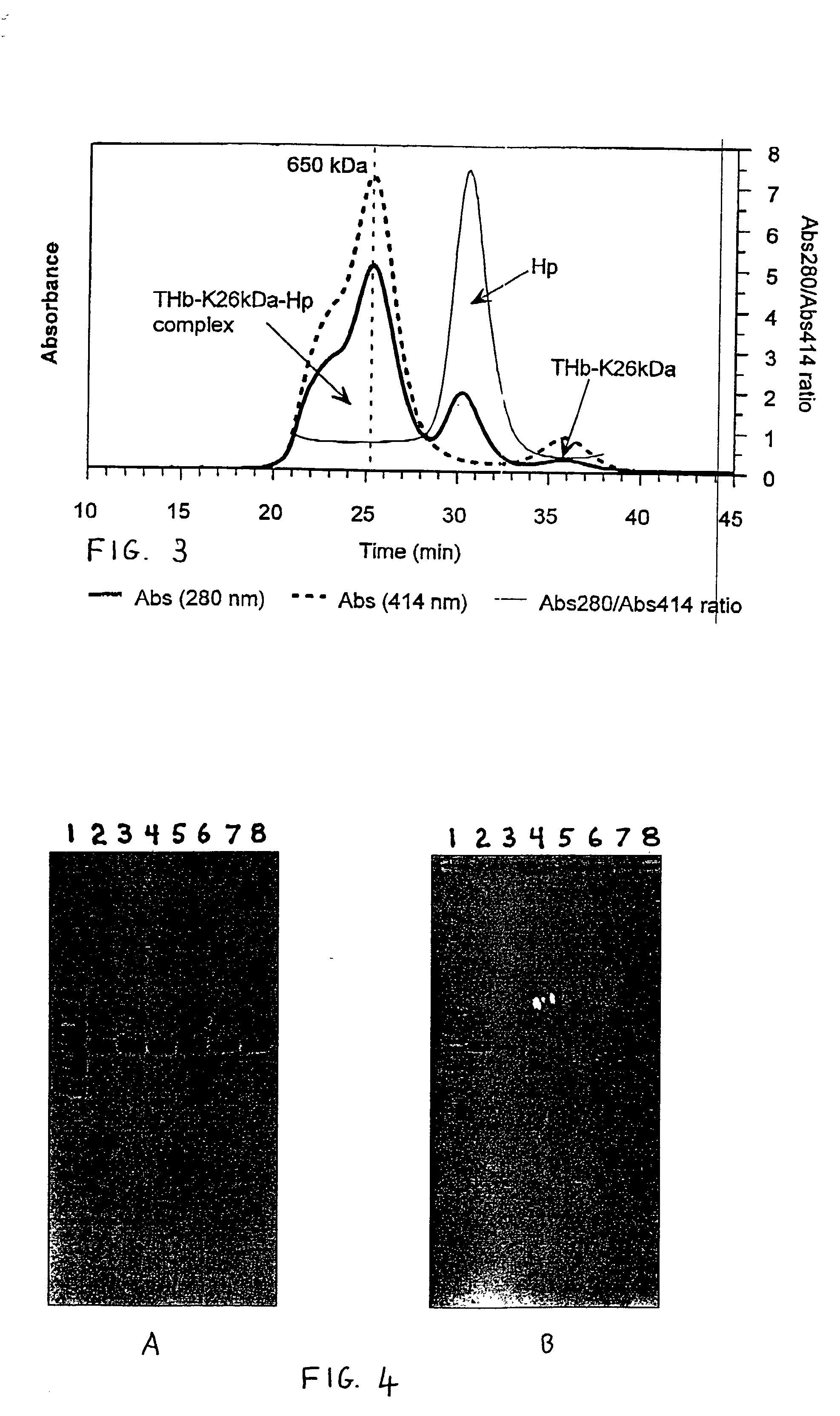
Complex Formation Between THb-K.sub.n and Haptoglobin 1-1
[0064] The following stock solutions were used for the preparation of the complexes: 1.74 mg / mL haptoglobin 1-1 (Hp) in water and 1.0 mg / mL solutions of the THb-K.sub.n (all THb-K.sub.n concentrations represent hemoglobin concentrations) in 50 mM sodium borate pH 9.0. Haptoglobin (14 .mu.L) was added to THb-K.sub.n in potassium phosphate pH 7.0 to give the following final concentrations: 0.12 mg / mL (1.22 .mu.M) haptoglobin and 0.19 mg / mL (2.9 .mu.M) THb-K.sub.n in 25 mM potassium phosphate pH 7.0 (200 .mu.L final volume). After incubation for 180 min. at room temperature, the samples were analyzed using SEC.
[0065] THb-K.sub.n complexes with haptoglobin 1-1: The formation of THb-K.sub.n complexes with haptoglobin can be followed using size exclusion chromatography (SEC). FIG. 2A shows the composition of the THb-K.sub.4kDa mixture with Hp after incubation at room temperature for 180 min. A new, high molecular weight peak appears...
example 3
DNA Binding to THb-K.sub.n and THb-K.sub.n-Hp. Gel Mobility Shift Assay
[0067] Gel mobility shift assays were conducted to evaluate the stoichiometry of binding of plasmid DNA (pCMVbeta) to the THb-K.sub.n conjugates. This gel electrophoretic method is based on the observation that the migratory properties of the DNA are altered upon binding protein. Neither proteins nor DNA-protein complexes in which protein constitutes a significant part of their mass enter 1% agarose gels. If mixtures with an increasing THb-K.sub.n to DNA ratio are analyzed, it is observed that the DNA band disappears at and above the ratio that corresponds to the stoichiometry of the complex. For each of the four conjugates and for the THb-K.sub.26kDa-Hp complex, solutions containing from 0.4 to 6400 ng of the conjugate (this weight based on the hemoglobin component) in 32 .mu.L of 20 mM HEPES pH 7.3 containing 150 mM NaCl were prepared. The plasmid DNA (560 ng in 28 .mu.L of 20 mM HEPES pH 7.3 containing 150 mM ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com