Drug combination for the treatment of viral diseases
a viral disease and drug combination technology, applied in the field of viral disease drug combination, can solve the problems of limited benefit of currently available antiretroviral agents which have undergone clinical evaluation, difficult to ascribe clinical failure solely, and major obstacles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
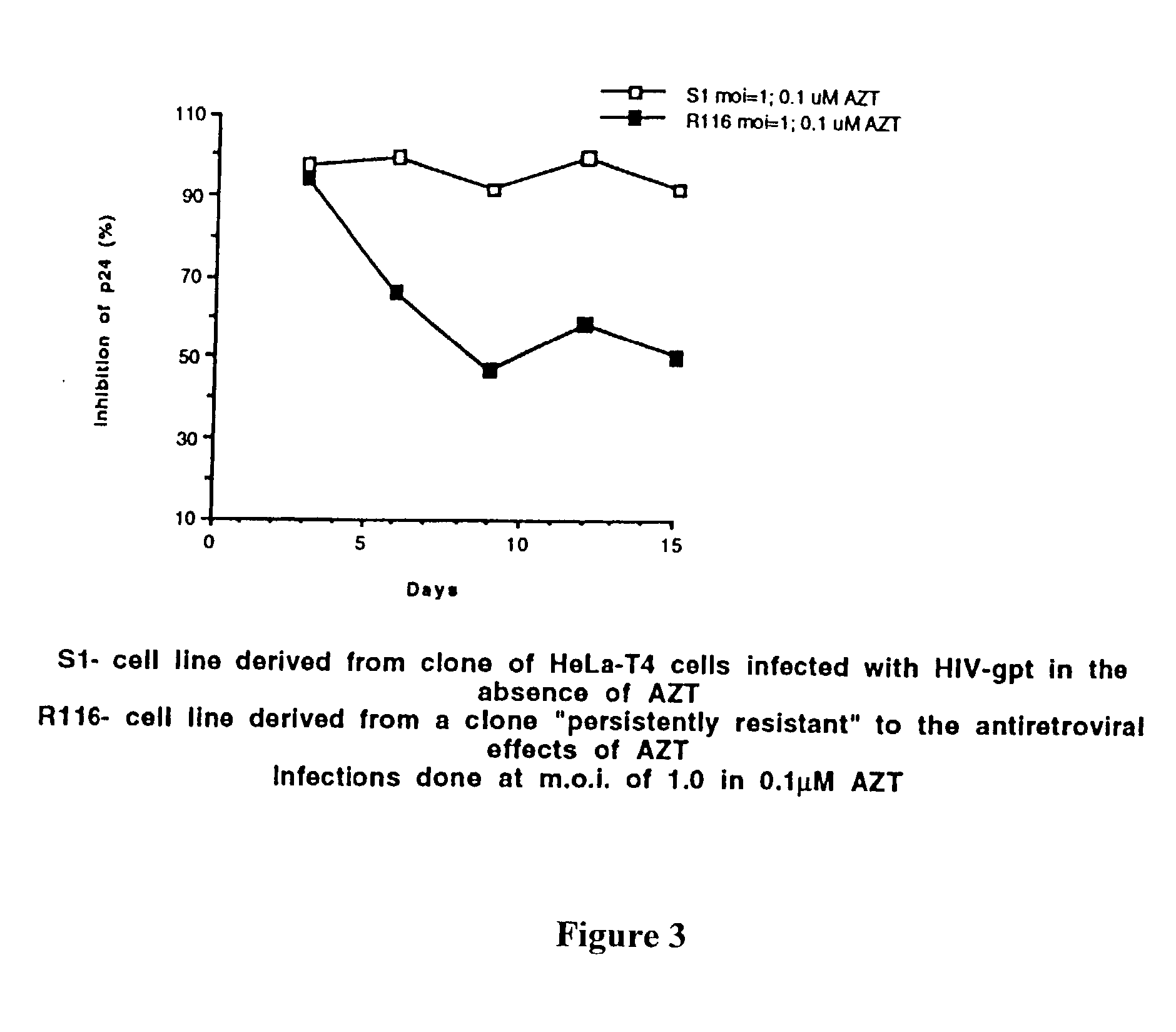
Sanctuary Growth of HIV in the Presence of AZT
Methods
Construction of Recombinant Proviral DNA
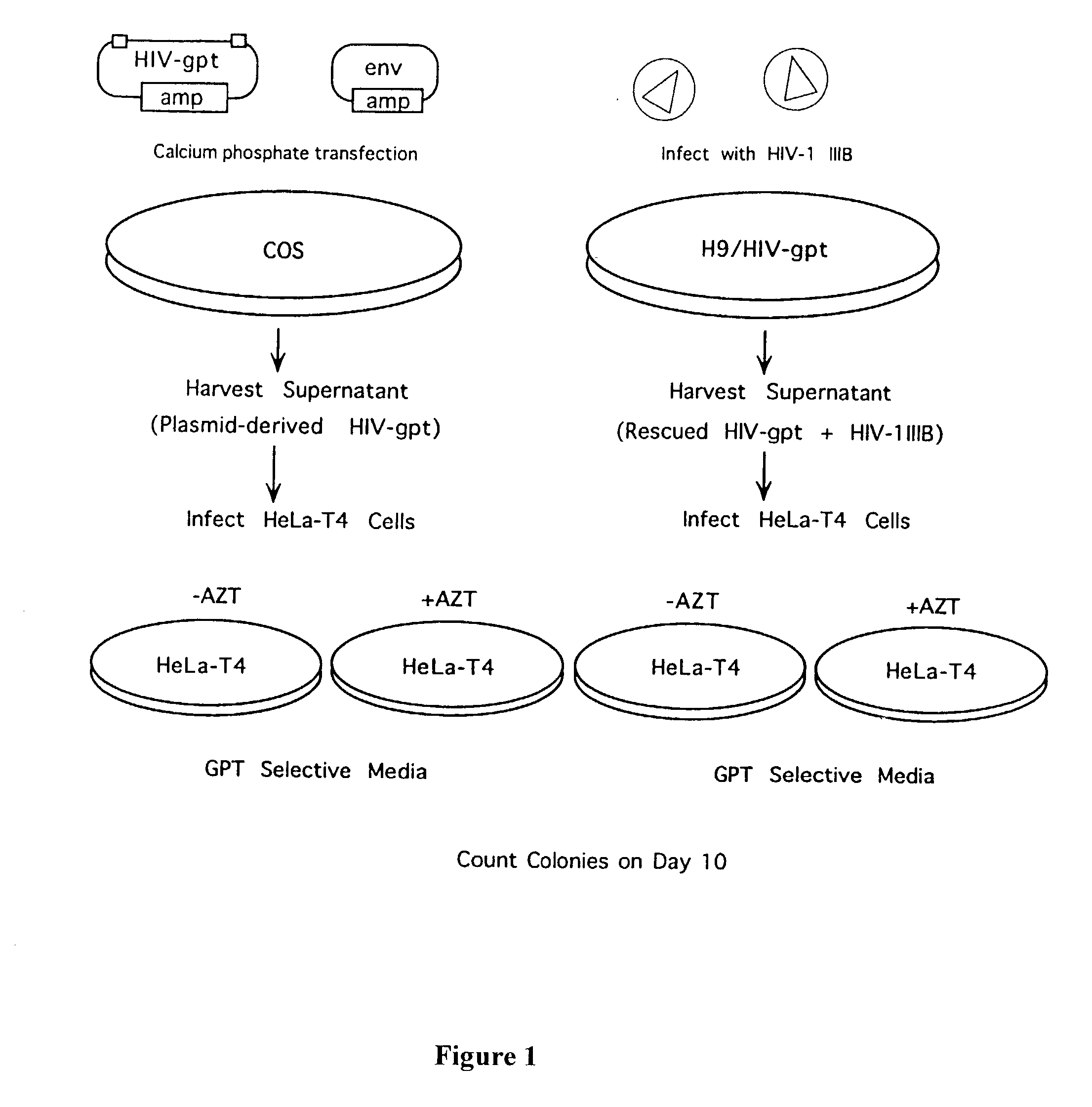
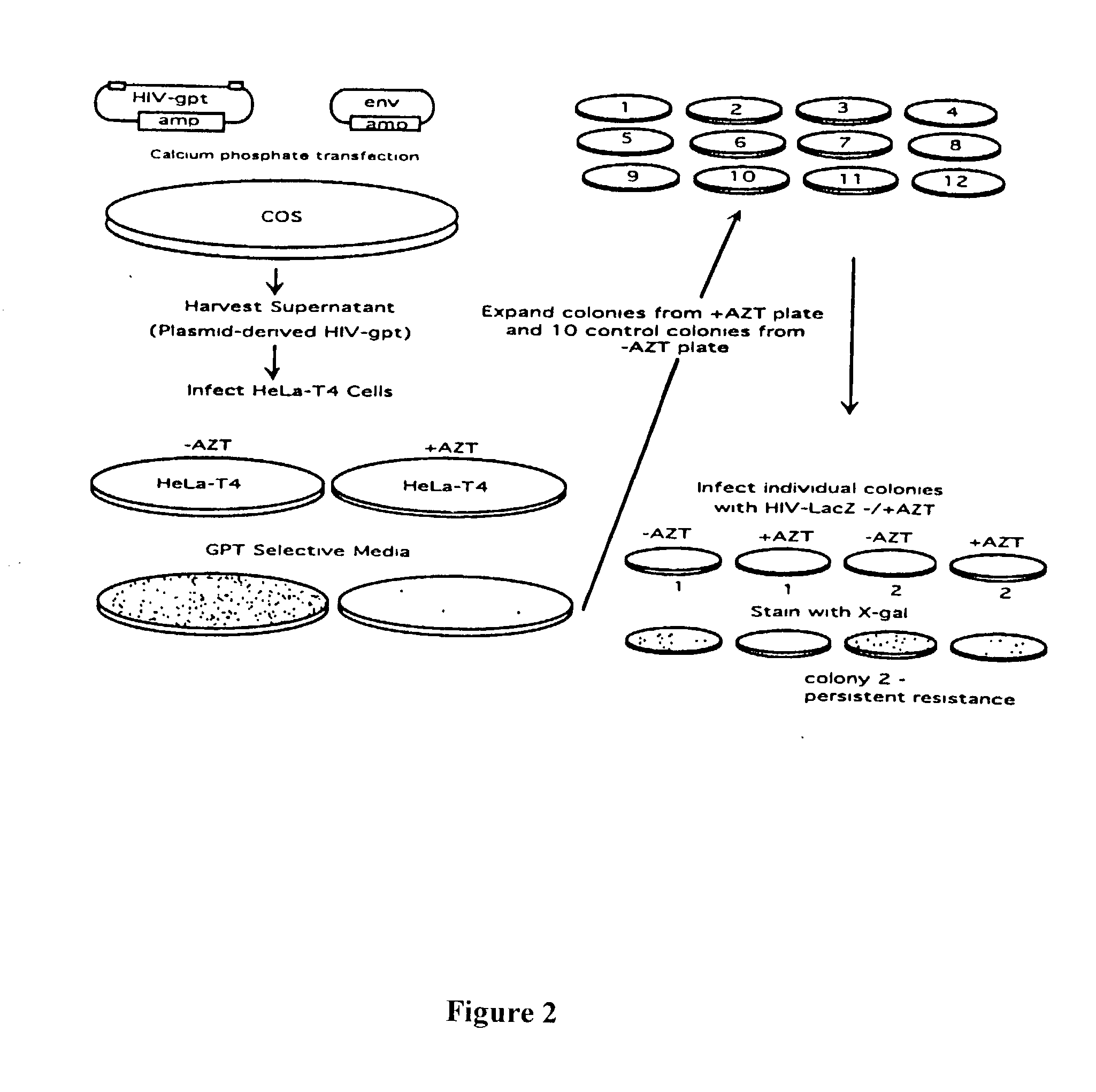
[0071] The HIV construct encoding LacZ has been described (26). It contains the LacZ gene driven by an SV40 promoter inserted into a large deletion in the HIV genome extending from the 5' end of the pol gene to the 3' end of the env gene. The HIV-gpt and HXB2env plasmids were kindly provided by Kathleen Page (University of California, San Francisco, Calif.) (18).
[0072] The HIV-gpt plasmid contains an HXB2 provirus into which an SV40 promoter gpt (E. coli guanine phosphoribosyl transferase) gene was inserted into the env region. The HXB2 env plasmid contains the HXB2 gp160 gene driven by an SV40 promoter.
Production of "Plasmid Derived" Recombinant Retroviruses
[0073] All transfections and cell culture were performed in an approved facility using BSL3 techniques. Plasmid DNA co-transfections into COS cells were performed as described by Page et al. (18). Supernatants from COS cells were collect...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com