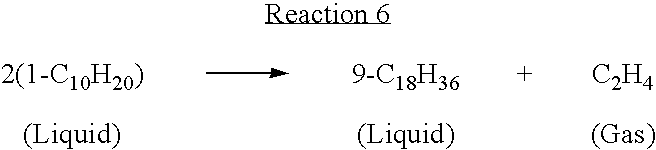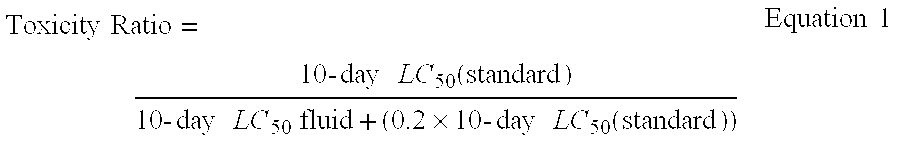Process for well fluids base oil via metathesis of alpha-olefins
a technology of well fluids and metathesis, which is applied in the direction of hydrocarbon preparation catalysts, wellbore/well accessories, sealing/packing, etc., can solve the problems of low production efficiency, high risk of waxy paraffin deposits in down hole equipment or risers, and low economic benefits of well fluids base oil
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
examples 1-5
[0073] In a glass slurry reactor a magnetic stirrer constantly agitated the reactor mixture so the 1-decene liquid and Catalyst A were in good contact during the one-hour reaction. Mineral oil bath provided homogeneous heating to the reactor, typically set at 60.degree. C. A septum on top of the reactor prevented the reaction mixture from contacting ambient air or moisture. For non-equilibrium operations during the reaction, nitrogen was introduced into contact with the reaction mixture through a syringe. A mass flow meter was always used to monitor the flow rate of the reaction off-gas in ml / min. Gas chromatography was used to analyze the 1-decene feed and the reaction products. Percent conversion of 1-decene was calculated by subtracting the weight % 1-decene in the product mixture from that in the feed, dividing by the amount in the feed, and then multiplying by 100. Selectivity was calculated by dividing the sum of the weight of C16, C17 and C18 olefins in the product mixture by...
examples 11-21
[0086] When subjected to isomerization conditions in the presence of an olefin isomerization catalyst, the metathesis liquids obtained using Catalyst A or Catalyst B acquire some unique and especially salient properties. The data of Table 5 show the unique properties obtained by using Catalyst B extrudates for self-metathesis of 1-decene followed by isomerization of the self-metathesis product of 1-decene. The metathesis was conducted using a continuous flow reactor with nitrogen gas flowing concurrently with the 1-decene feed. The ratio of nitrogen purge flow to 1-decene was 1.5 g nitrogen to 1 g 1-decene. The beginning reactor temperature was 40.degree. C., the WHSV was 1, and a pressure of 1 atmosphere was employed. The initial conversion rate for fresh catalyst was always 90% with high selectivity (95-97% without accounting for ethylene). As the catalyst deactivates the conversion fell to .about.75% at which time reactor temperature was increased to raise conversion rate but sel...
example 24
[0094] Example 24 was done to illustrate the cross-metathesis embodiment of the inventive process. For Example 24, 1-octene and 1-decene were subjected to cross-metathesis in a manner similar to previous examples of self-metathesis of 1-decene. Experimental conditions were: 10 grams of catalyst A in a tubular fixed bed reactor with upflow of the continuous liquid feed, feed was 30 wt % 1-octene and 70 wt % 1-decene, 1.0 WHSV (weight hourly space velocity), co-current nitrogen purging using 1.5 g N.sub.2 per g of liquid feed, and a metathesis reactor temperature of 40.degree. C. The reactor product sample was taken after 24 hours of reaction time. The resulting compositions are given in Table 9 below.
9TABLE 9 Cross Metathesis of 1-octene and 1-decene Feed, Product, Species wt % wt % light gases 0 10 C2 to C7 olefins C8 olefins 30 2.9 C10 olefins 70 6.5 C11 olefins 0 0.3 C12 olefins 0 0.1 C13 olefins 0 0.3 C14 olefins 0 7.9 C15 olefins 0 2.5 C16 olefins 0 32.9 C17 olefins 0 1.8 C18 ol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| metathesis temperature | aaaaa | aaaaa |
| pour point temperatures | aaaaa | aaaaa |
| pour point temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com