Process for discriminating and counting erythroblasts
a technology of erythroblasts and counting methods, applied in the field of counting erythroblasts, can solve the problems of inability to accurately determine erythroblasts, and complicated pretreatment of blood for examination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0070] First, reagents having the following compositions were prepared.
[0071] Fluorescent labeled antibody:
1 FITC labeled anti-CD45 antibody First reagent fluid (pH 3.0, osmotic pressure 16 mOsm / kg .multidot. H.sub.2O): Buffering agent Citric acid monohydrate, 2.10 g / l Disodium hydrogenphosphate, 0.56 g / l Nucleotide fluorescent dye Propidium iodide, 100 mg / l Purified water Second reagent fluid (pH 7.5, osmotic pressure 420 mOsm / kg .multidot. H.sub.2O): Buffering agent Sodium dihydrogenphosphate 0.95 g / l dihydrate, Disodium hydrogenphosphate, 6.24 g / l Osmolarity compensating Sodium chloride, 10.2 g / l agent Purified water
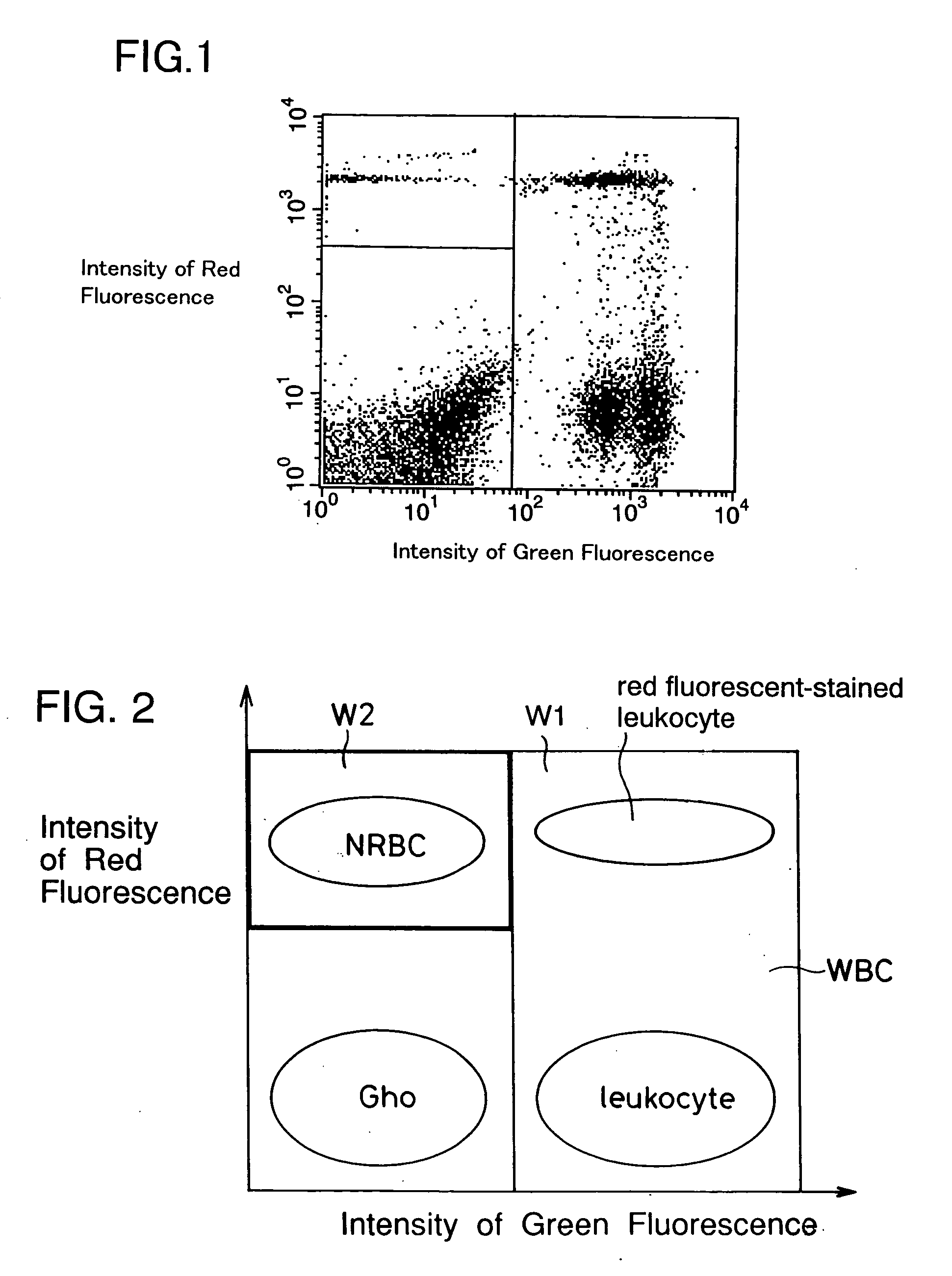
[0072] Fifty (50) .mu.l of blood from a patient was added to peripheral blood to produce a hematologic sample. These bloods had been treated with an anticoagulant. Ten (10) .mu.l of the above FITC labeled anti-CD45 antibody were added to the hematologic sample. This mixture was incubated at room temperature for about 15 minutes. Here, the blood containing erythroblast...
example 2
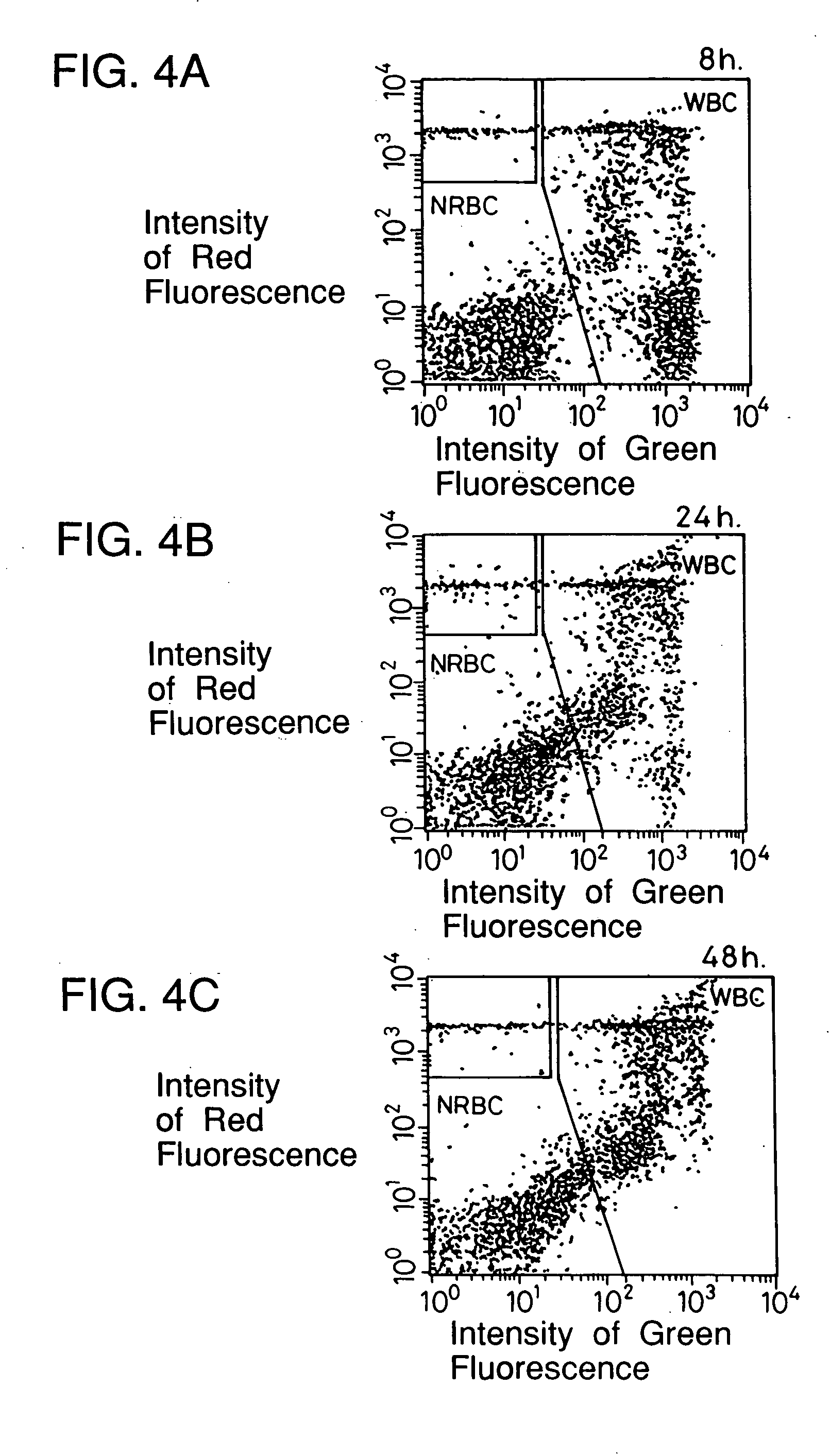
[0080] Blood containing erythroblasts from two patients other than the patient in Example 1 and peripheral blood (preserved at room temperature for 8 hours, 24 hours, 48 hours after collection) were examined about leukocytes and erythroblasts in the same manner as in Example 1. The results are shown in FIGS. 4A to 4C and FIGS. 5A to 5C.
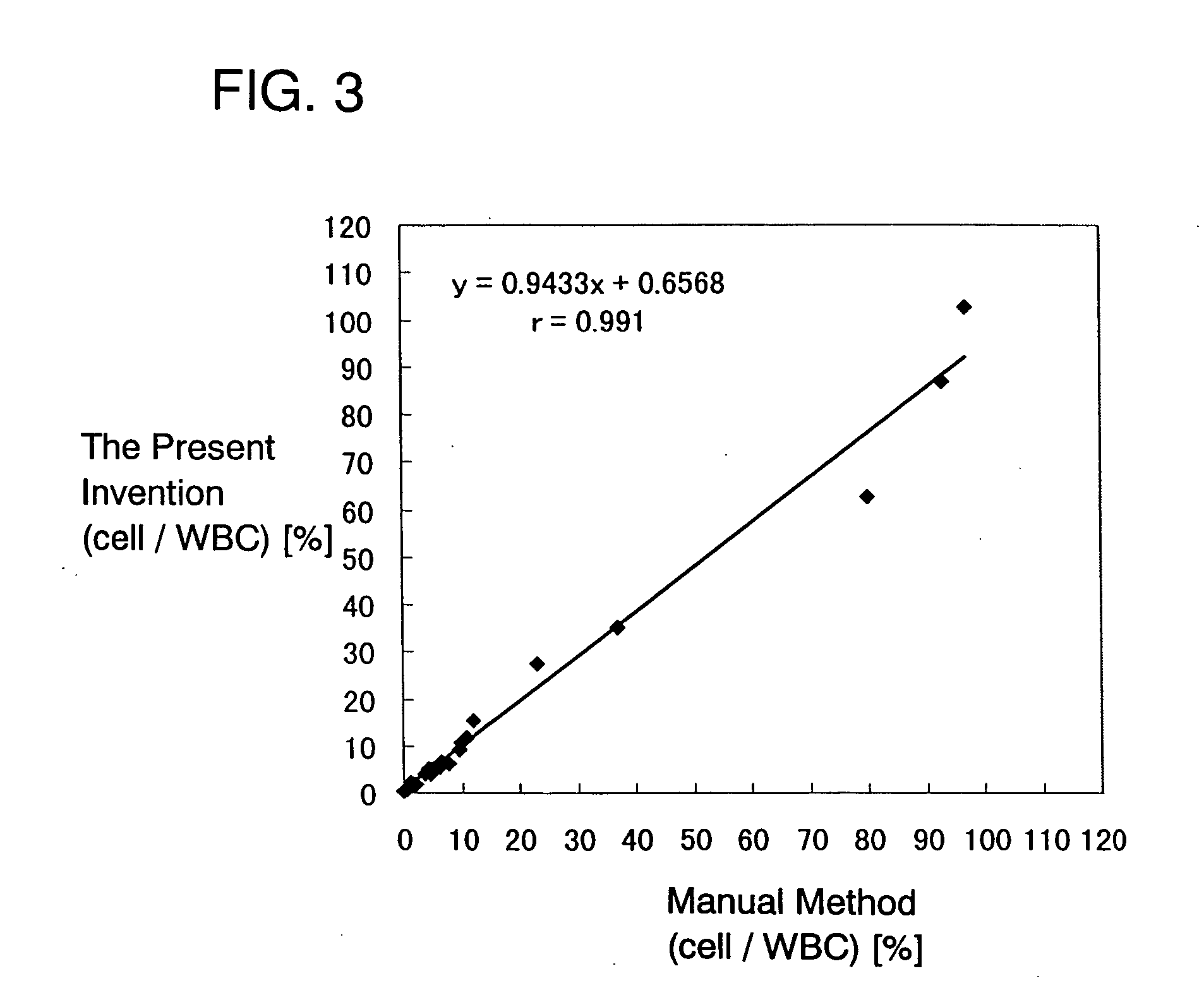
[0081] The ratio of the erythroblasts to the leukocytes was also calculated for each of the samples. The results are shown in Table 1.
2 TABLE 1 after 8 hours after 24 hours after 48 hours Sample 1 3.6% 3.6% 3.6% Sample 2 6.1% 6.1% 6.7%
[0082] FIGS. 4A to 4C and 5A to 5C and Table 1 show that the passing of time hardly affects the results of examination according to the present invention.
example 3
[0083] Reagents having the following compositions were prepared.
[0084] Fluorescent labeled antibody:
3 FITC labeled anti-CD45 antibody First reagent fluid (pH 3.0, osmotic pressure 16 mOsm / kg): Buffering agent Citric acid monohydrate, 2.10 g / l Disodium hydrogenphosphate, 0.56 g / l Nucleotide fluorescent dye Propidium iodide, 1 mg / l Purified water Second reagent fluid (pH 7.5, osmotic pressure 420 mOsm / kg): Buffering agent Sodium dihydrogenphosphate 0.95 g / l dihydrate, Disodium hydrogenphosphate, 6.24 g / l Osmolarity compensating Sodium chloride, 10.2 g / l agent Purified water
[0085] Fifty (50) .mu.l of blood from a patient was mixed with ten (10) .mu.l of the above FITC labeled anti-CD45 antibody. These bloods had been treated with an anticoagulant. This mixture was incubated at room temperature for about 15 minutes.
[0086] Then, 500 .mu.l of the first reagent fluid were added to the mixture, which was incubated at room temperature for about 30 seconds. To the resulting mixture, 1000 .mu....
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com