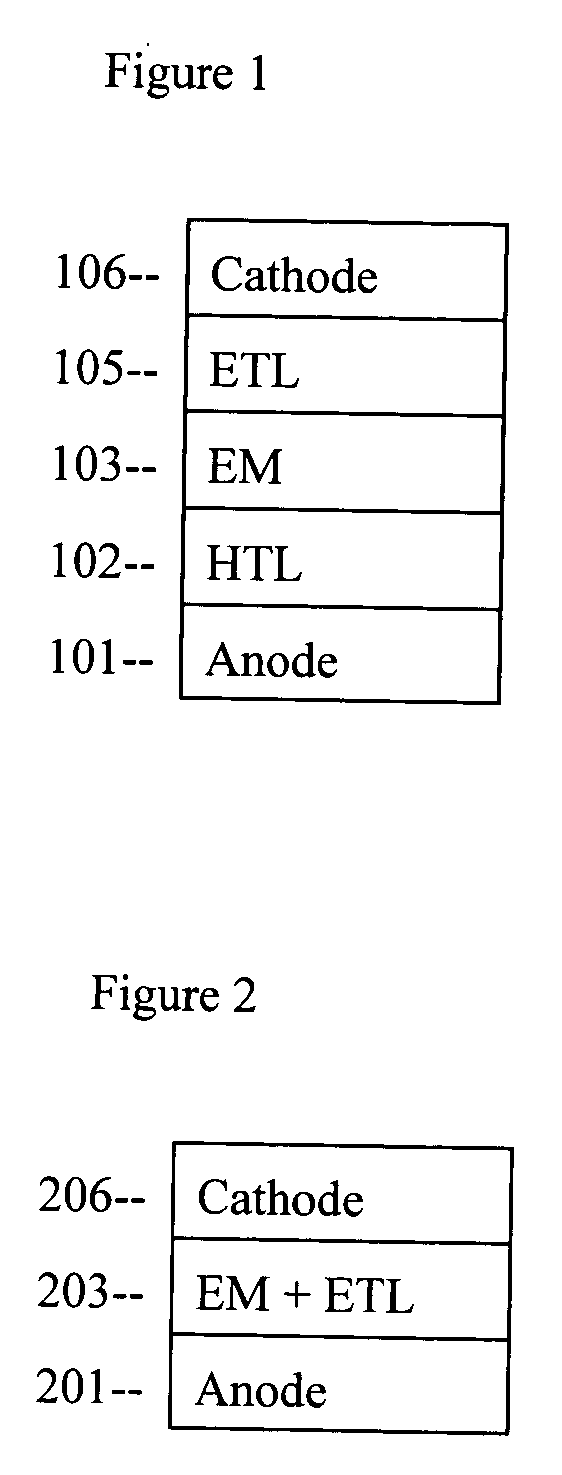
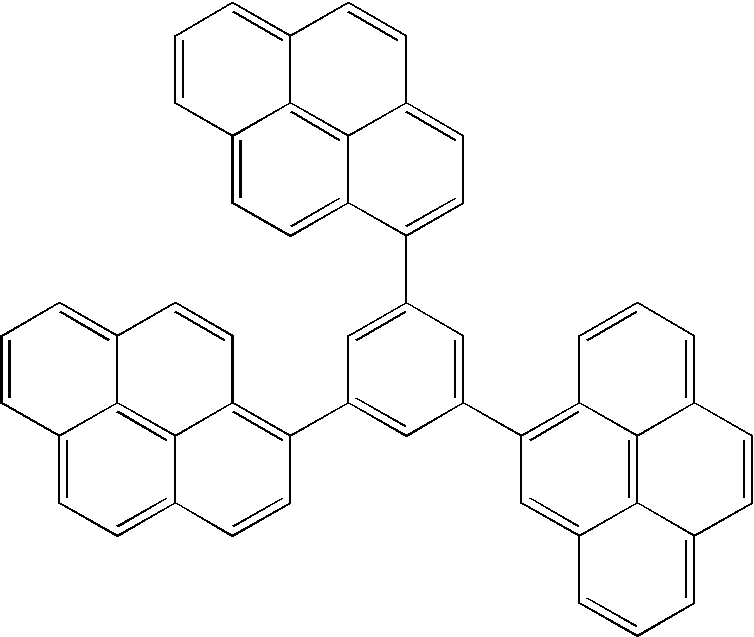
Organic electroluminescent device based on pyrene derivatives
a technology of pyrene derivatives and electroluminescent devices, which is applied in the direction of discharge tube luminescnet screens, natural mineral layered products, other domestic articles, etc., can solve the problems of tainted blue emission of these materials, and achieve the effect of improving blue emission purity and reducing aggregation problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
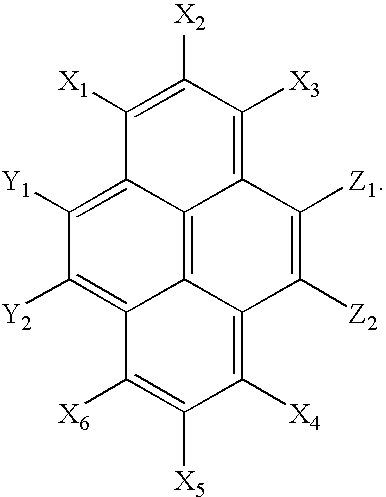
[0035] The pyrene based compound of the present invention has the following general formula (I):
[0036] wherein Z1 represents a hydrogen atom, deuterium atom, oxygen atom, silicon atom, selenium atom, substituted or unsubstituted aryl group, substituted or unsubstituted heteroaryl group, substituted or unsubstituted aryl amine or a combination thereof, and Z2 represents a hydrogen or deuterium atom; [0037] wherein one of Y1 and Y2 represents a hydrogen atom, deuterium atom, oxygen atom, silicon atom, selenium atom, a substituted or unsubstituted aryl group, substituted or unsubstituted heteroaryl group, substituted or unsubstituted aryl amine or a combination thereof, and the other of Y1 and Y2 represents a hydrogen or deuterium atom; [0038] wherein X1 through X6 independently represent hydrogen atoms, deuterium atoms, alkyl groups or aryl groups, and at least one of X1 through X6 represents a bulky alkyl group or bulky aryl group; and [0039] wherein at least one of X1 through X6, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| transparent | aaaaa | aaaaa |
| work function | aaaaa | aaaaa |
| power efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com