Liquid crystal based analyte detection
a liquid crystal and analyte technology, applied in the field of liquid crystal based analyte detection, can solve the problems of high cost, high complexity and/or complexity of methods with the highest sensitivity (real-time pcr, tissue culture, electron microscopy), and high cost, and achieve the effect of less sensitivity, high cost, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Immobilization of Antibodies on Substrates
This example describes different methods for immobilizing antibodies on substrate. Five different immobilization strategies were evaluated:
1) HEXA: adsorption of Protein A, then the West Nile Virus monoclonal antibodies (WNV Mabs) onto a hydrophobic monolayer formed from CH3(CH2)15SH (HEXA) on the surface of a gold film. The surface was blocked with BSA after immobilization of the antibody.
2) SPDP: covalent attachment of WNV Mabs to a monolayer formed from 2-mercaptoethylamine (2-MEA) on a gold film by using the sulfhydryl-reactive (protein) and amine-reactive (monolayer) heterobifunctional cross-linker N-succinimidyl 3-(2-pyridyldithio)propionate (SPDP). The surface was blocked with BSA after immobilization of the antibody.
3) PMPI: covalent attachment of Ras polyclonal antibodies (Ras Pabs) to a monolayer formed from 11-mercaptoundecanol (11-MU) on a gold film by using a sulfhydryl-reactive (protein) and hydroxyl-reactive (monolayer...
example 2
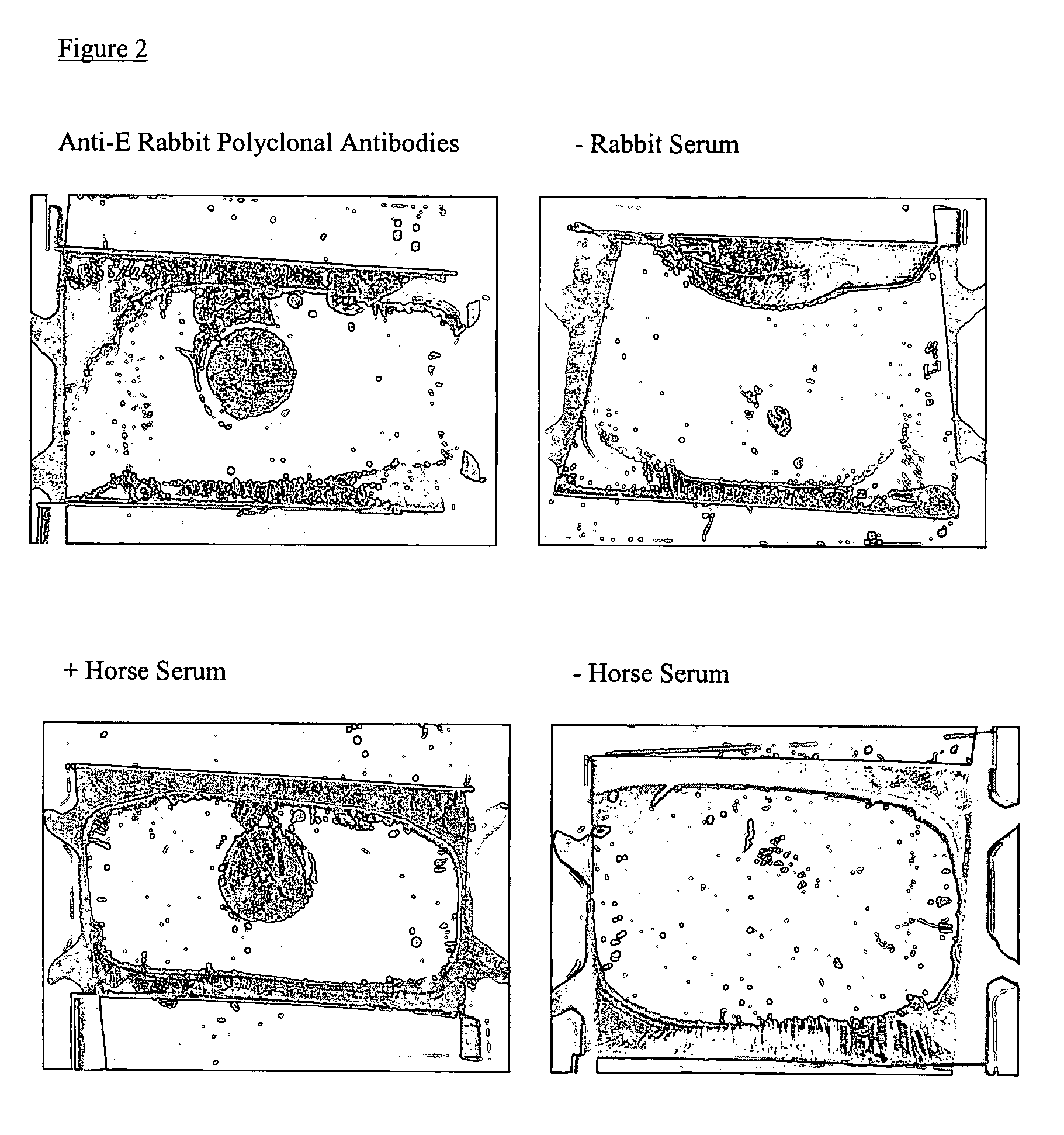
Detection of West Nile Virus
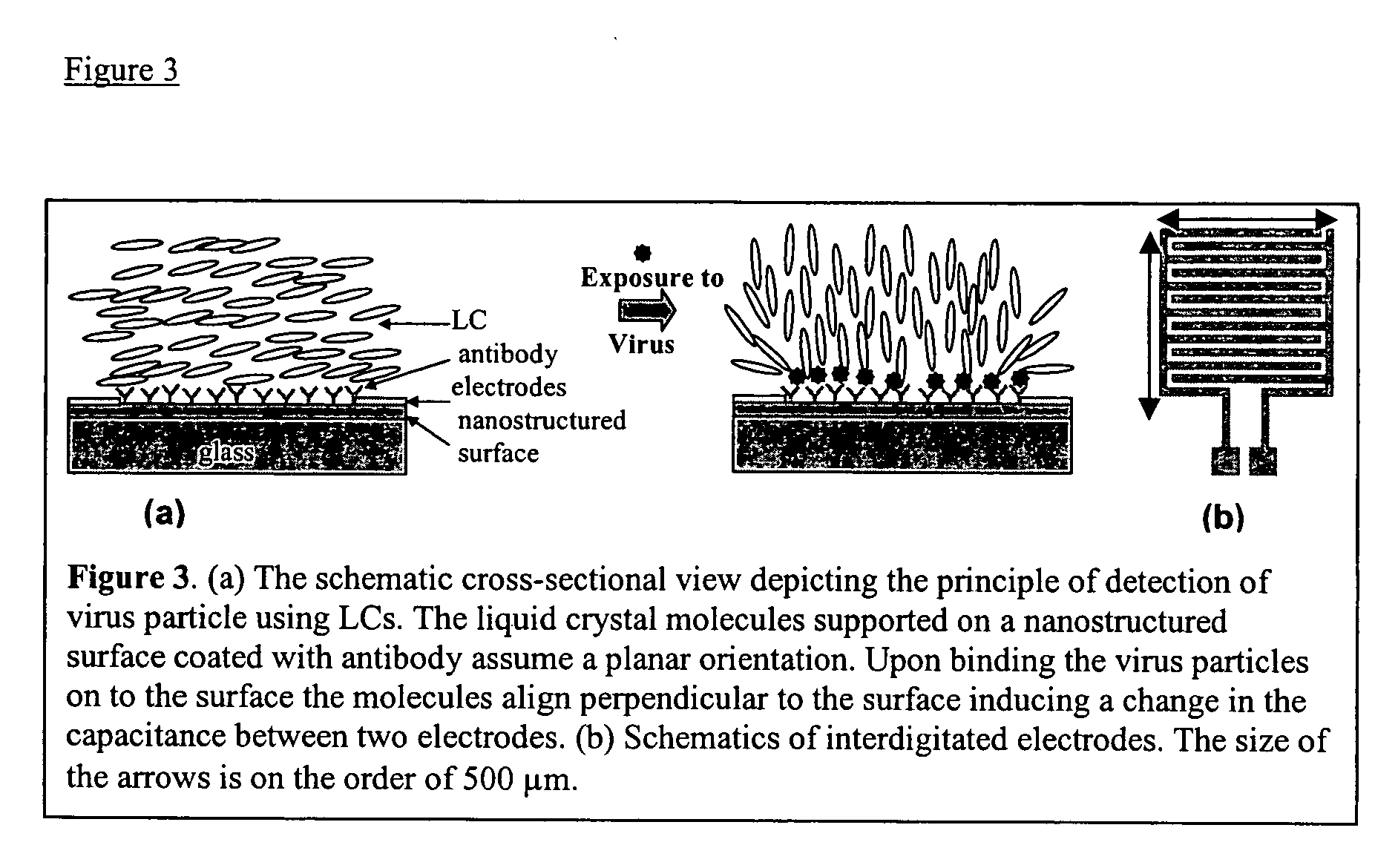
The detection of viruses with liquid crystal assays in which the detection region comprises topographical features has been described in WO 01 / 61357. These types of surfaces in combination with liquid crystals were successfully used to report the presence of West Nile Virus (WNV) captured on the surface of such substrates. Surprisingly, however, it has now been found that the reporting mechanism does not require the topography on the surface. This unexpected outcome substantially simplifies the fabrication of substrates for detection of viruses using liquid crystals. As described below, it has been demonstrated that this reporting mechanism can be applied to different viruses.
Antibodies to WNV were deposited onto the surface of molded polyurethane replicas. The micromolded replicas had a pitch of 400 nm and a depth of 54 nm. A drop of aqueous solution containing WNV was deposited onto the surface of the polymeric replica. The solutions contained 108.4...
example 3
Optimization of Antibody Immobilization
Different methods of immobilizing the antibody were investigated to determine the procedure which would give the best results. Briefly, polyurethane substrates were functionalized with (a) 1 uM WNV monoclonal antibodies, (b) 5 uM WNV monoclonal antibodies, and (c) 1 mg / ml Protein A first, then 1 uM WNV monoclonal antibodies. All functionalized substrates were then incubated with the WNV stock. The results showed that substantially the same homeotropic response is observed when the polyurethane is functionalized with 1 uM or 5 uM antibody, and also when the substrate is first incubated with Protein A (molecule which correctly orients the antibody), and then functionalized with 1 uM antibody. These results indicate that a strong homeotropic response can be obtained with a lower concentration of antibody, with or without Protein A. The current method involves coating the entire substrate with 1 mg / ml Protein A, and subsequently immobilizing anti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelengths | aaaaa | aaaaa |
| wavelengths | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com