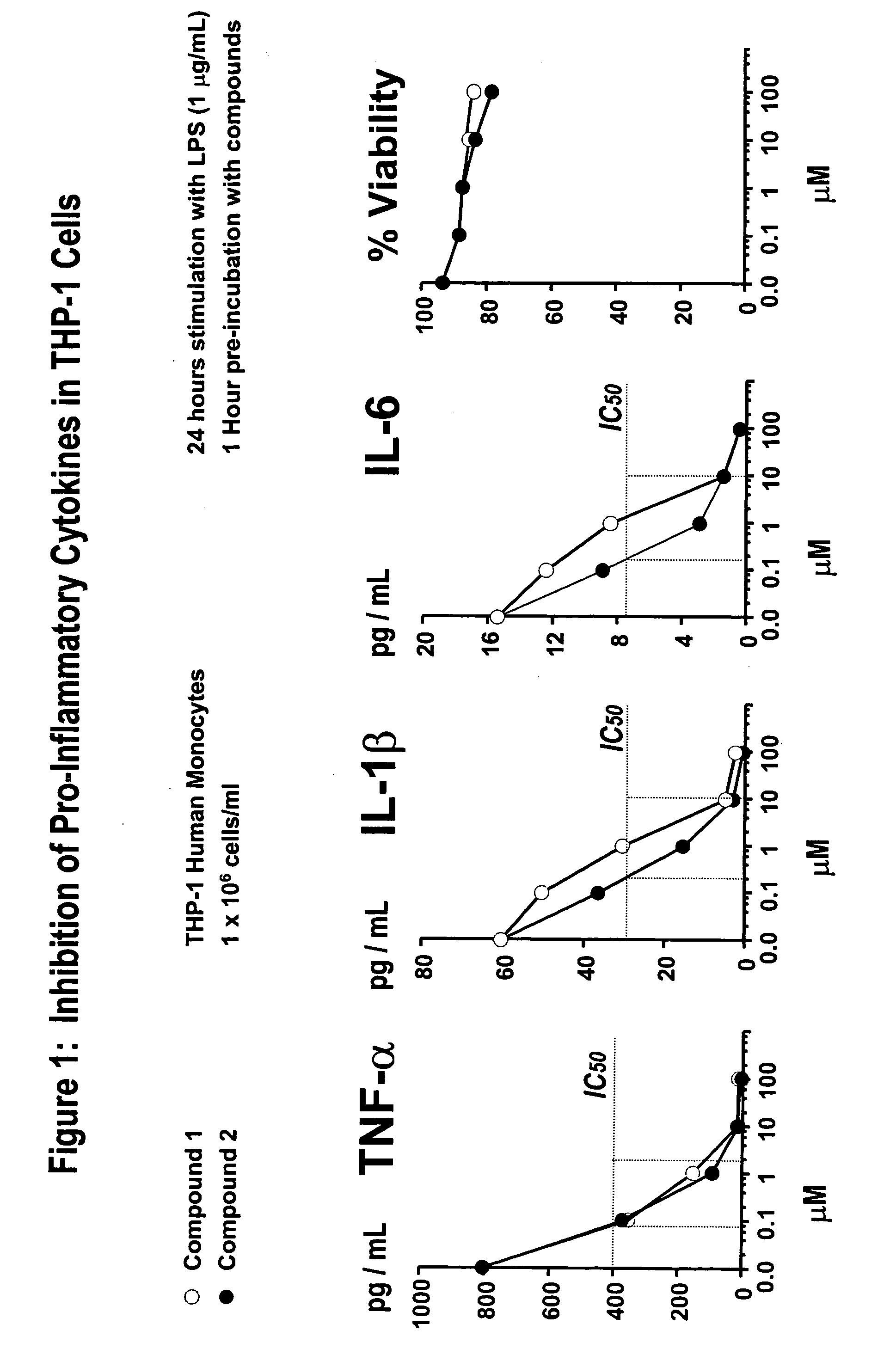
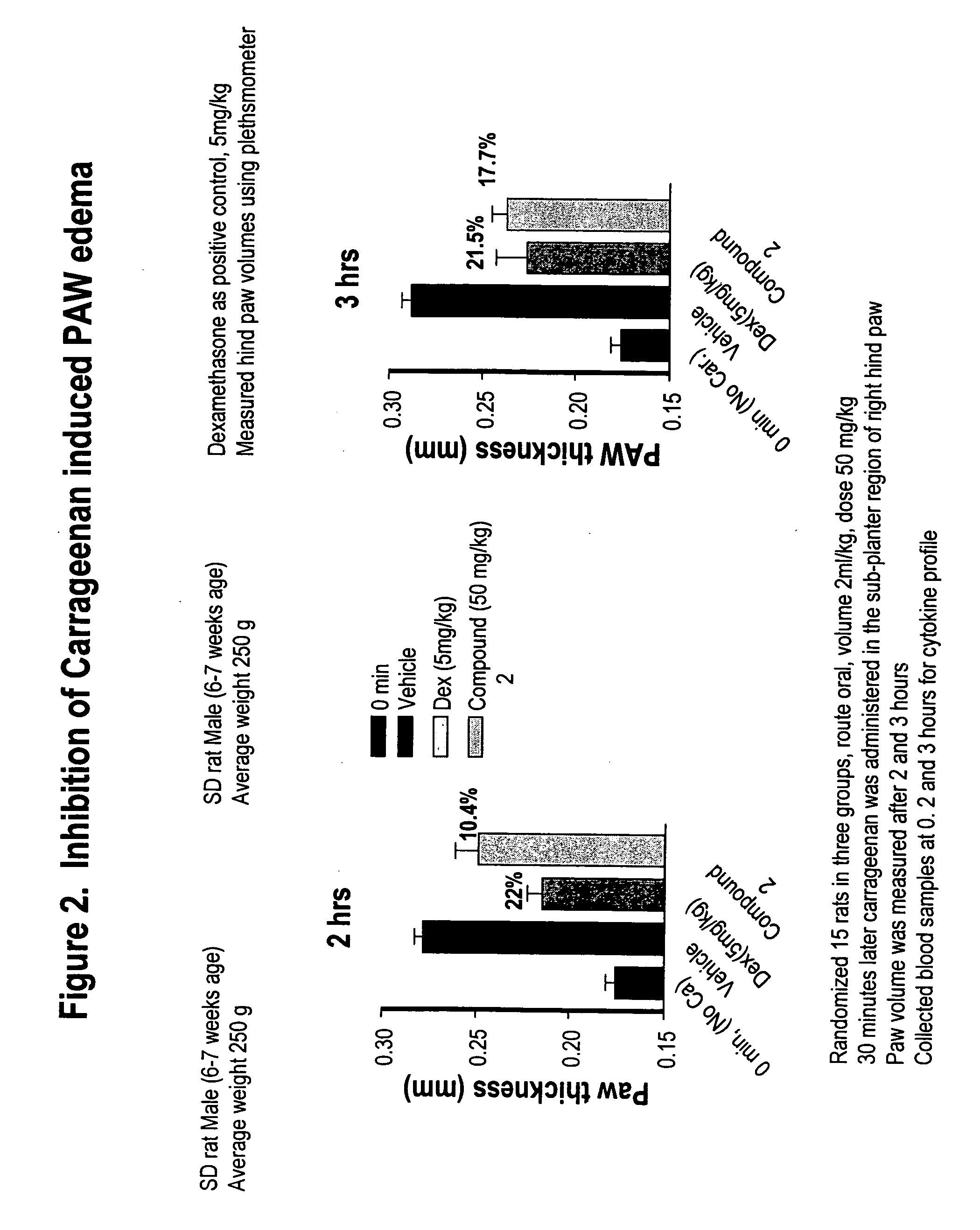
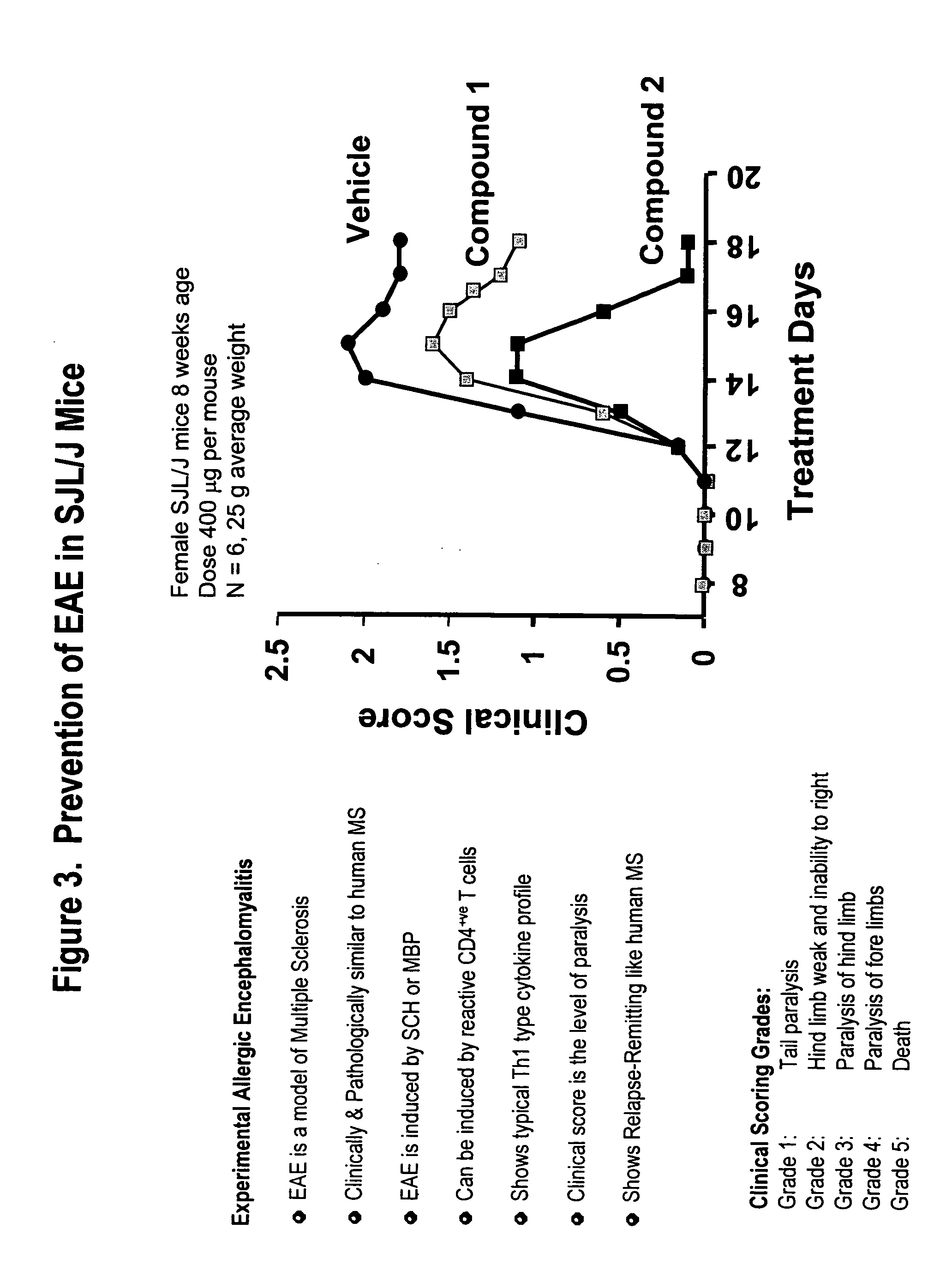
Amino acid phenoxy ethers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 5-[4-(4-(2-amino-2-methoxycarbonylethyl)phenoxy)benzyl]-thiazolidin-2,4-dione hydrochloride salt
[0215]
Step (i)
Preparation of methyl-2-[(t-butoxycarbonyl)amino]-3-[-(4-formylphenoxy)phenyl]propanoate
[0216] To a suspension of fresh sodium hydride (0.813 g, 33.9 mmol) in dry DMF (20 ml) under nitrogen atmosphere was added the solution of methyl-2-[(t-butoxycarbonyl)amino]-3-(4-hydroxyphenyl)propanoate (10 g, 33.9 mmol) in DMF (20 ml) slowly. The mixture was stirred for 15 minutes. 4-Fluorobenzaldehyde (4.20 g, 33.9 mmol) was added and the mixture was heated to 80° C. After completion of the reaction, the solvent was removed by high vacuum and the mixture was quenched with addition of saturated aqueous ammonium chloride. The mixture was extracted with ethyl acetate (3×50 ml). After washing with brine and dried on anhydrous sodium sulfate, the solvent was evaporated and the product was purified with flash column from eluent of hexanes: ethyl ether; 12 / 30 to 12 / 22 to aff...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Bond | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com