SH2 domain binding inhibitors
a technology of sh2 domain and inhibitor, which is applied in the field of macrocyclic peptides, can solve the problems of high affinity binding loss, suffering and possibly death,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
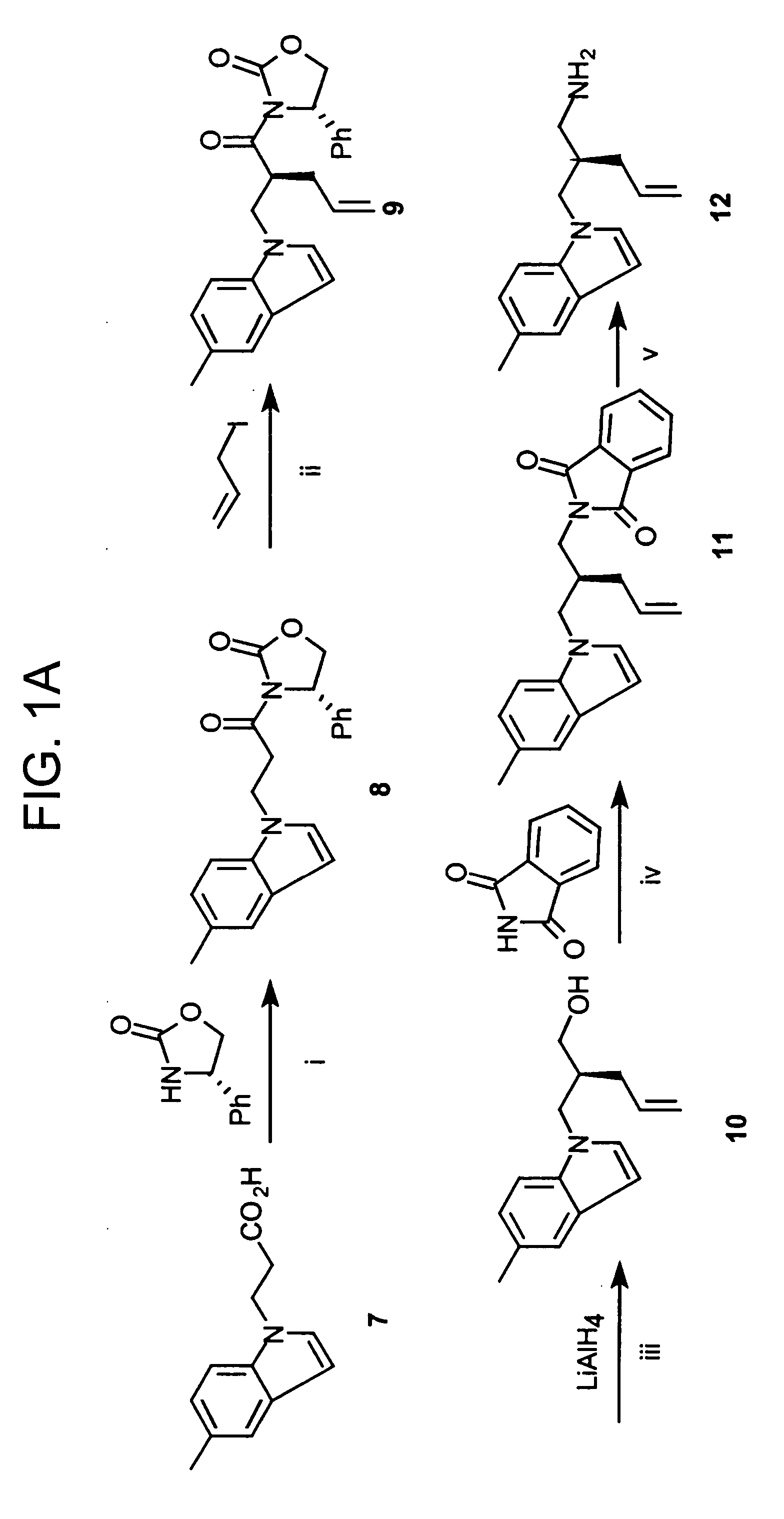
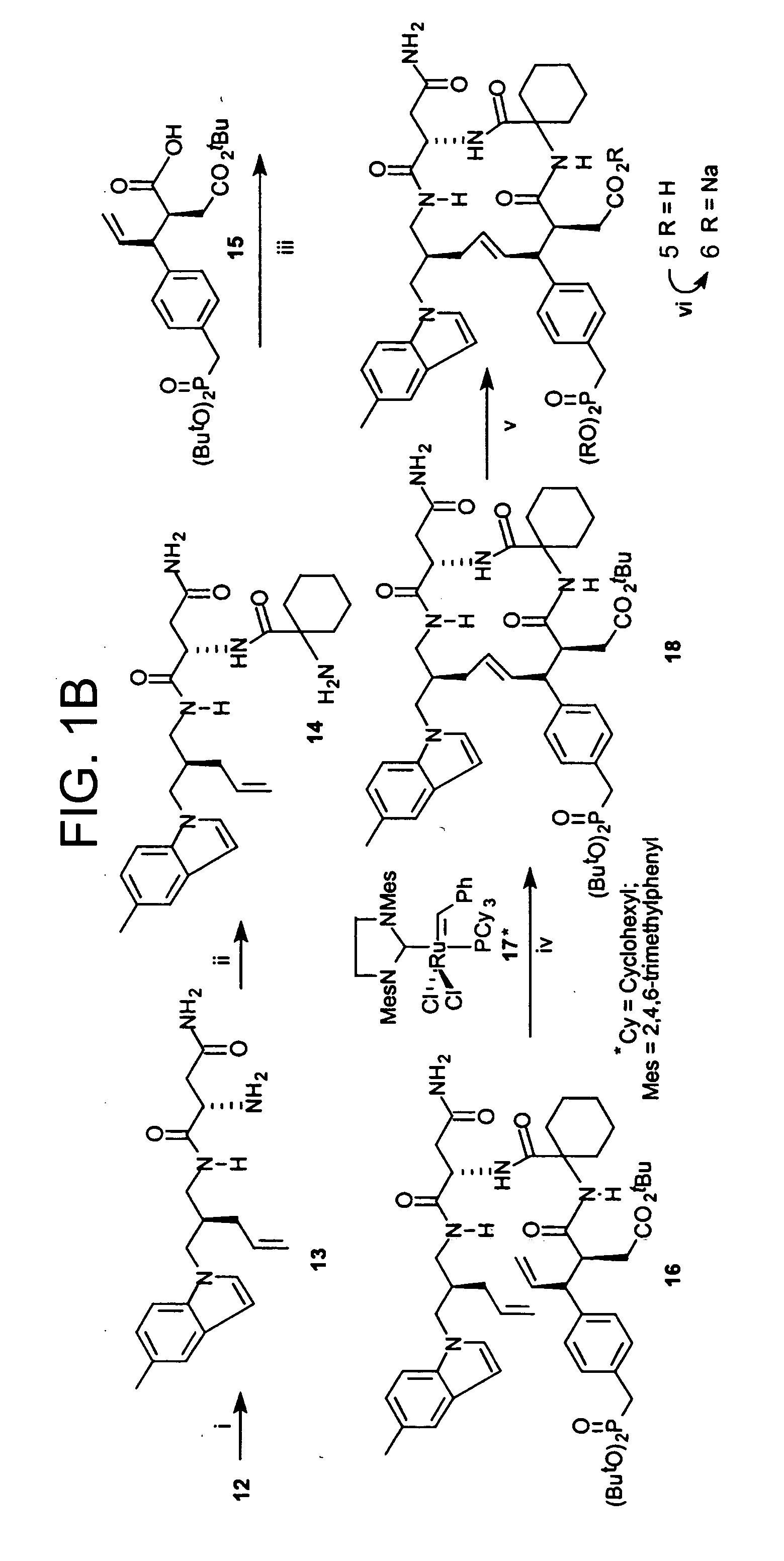
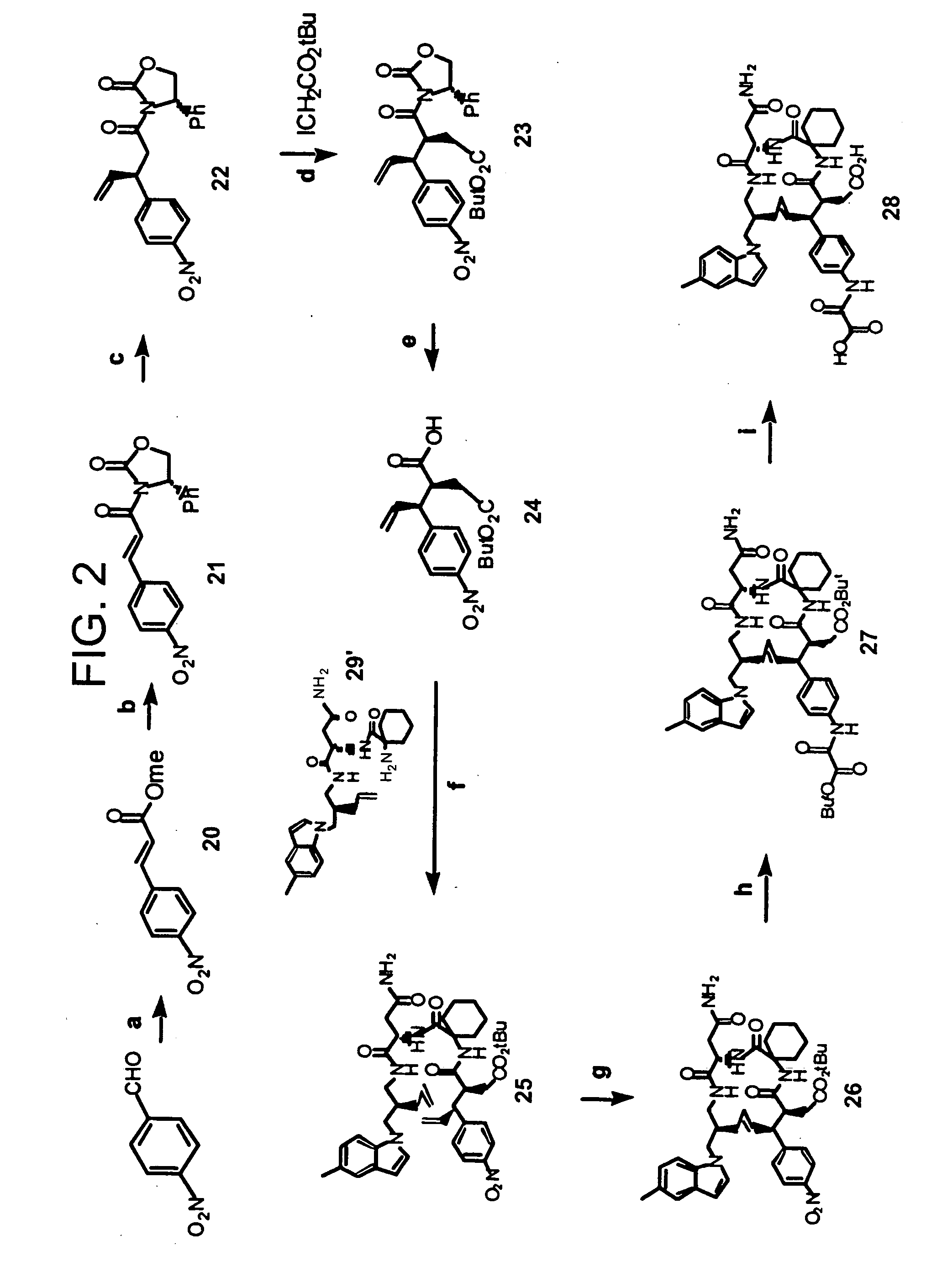
[0060] This Example illustrates methods of preparing compounds in accordance with an embodiment of the present invention. The methods are illustrated schematically in FIGS. 1-6.
[0061] Compounds 5-6 are prepared as shown in FIGS. 1A-B.
[0062] (4S)-3-[3-(5-methylindolyl)propanoyl]-4-phenyl-1,3-oxazolidin-2-one (8). To the suspension of (4S)-4-phenyl-1,3-oxazolidin-2-one (6.85 g, 42 mmol) in anhydrous THF (130 mL) was added BuLi (26.3 mL, 42 mmol) at −78° C. and the mixture was stirred under argon (0.5 h). To the above suspension was added a solution of active ester prepared by reacting 3-(5-methylindolyl)propanoic acid 7 (10.23 g, 50.39 mmol) in anhydrous THF (130 mL) and trimethylacetyl chloride (5.10 g, 50.39 mmol) in the presence of N-methyl morphine (6.06 g, 50.39 mmol) at 0° C. under argon, followed by stirring at −78° C. (1 h). The combined reaction mixture was stirred at −78° C. for an additional 2 h, then the solution was warmed to room temperature and stirred overnight. The ...
example 2
[0116] This example illustrates some of the properties of compounds in accordance with an embodiment of the invention.
[0117] Binding affinities were determined as follows. Surface Plasmon Resonance (SPR) Determination of Grb2 SH2 Domain-Binding Kd Values. Binding experiments were performed on a BIACORE S51 instrument (Biacore Inc., Piscataway N.J.). All Biotinylated Grb2 SH2 domain protein (b-Grb2) was expressed and purified (Protein Expression Laboratory and The Protein Chemistry Laboratory, SAIC-Frederick). The b-Grb2 was immobilized onto carboxymethyl 5′ dextran surface (CM5 sensor chip, Biacore Inc.) by amine coupling. The lyophilized b-Grb2 was reconstituted in fifty percent DMSO in H2O to make a stock solution of 1 mg / mL and stored at −80° C. A 1:12.5 dilution of b-Grb2 was used for immobilization and prepared by dilution in acetate buffer pH-5.0, with 5% DMSO. 1×PBS (phosphate buffered saline, pH 7.4) was used as the running buffer.
[0118] An immobilization wizard was used t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Stereoisomer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com