Implantable product with improved aqueous interface characteristics and method for making and using same
a technology of aqueous interface and product, which is applied in the field of implantable medical devices, can solve the problems of difficult to properly visualize using certain remote visualization techniques, poor initial visualization following, and difficulty in making devices made from microporous polymers, etc., to achieve rapid and accurate visualization, eliminate air interference issues, and increase the rate of air displacement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Process for Coating a Septal Occluder
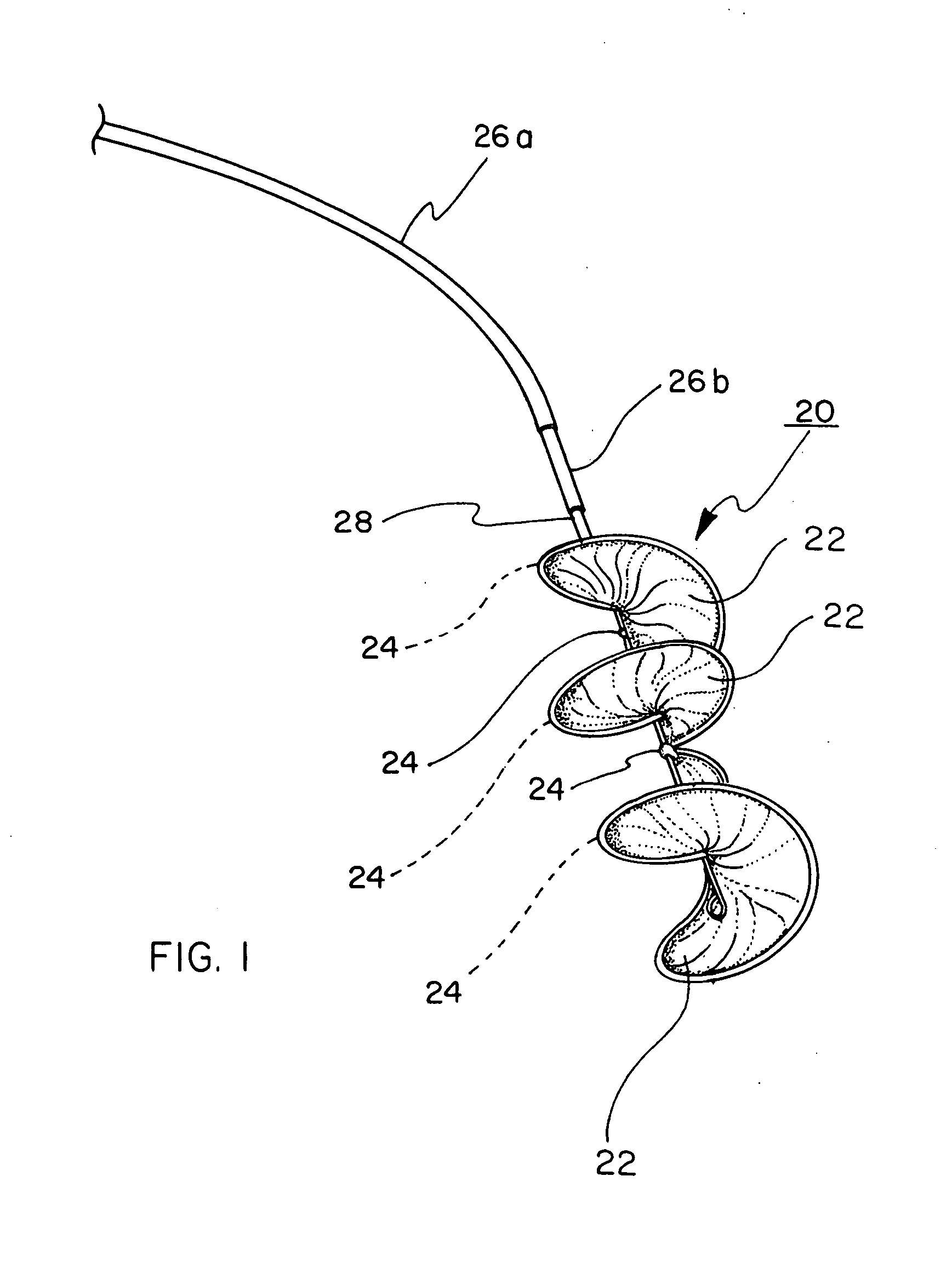
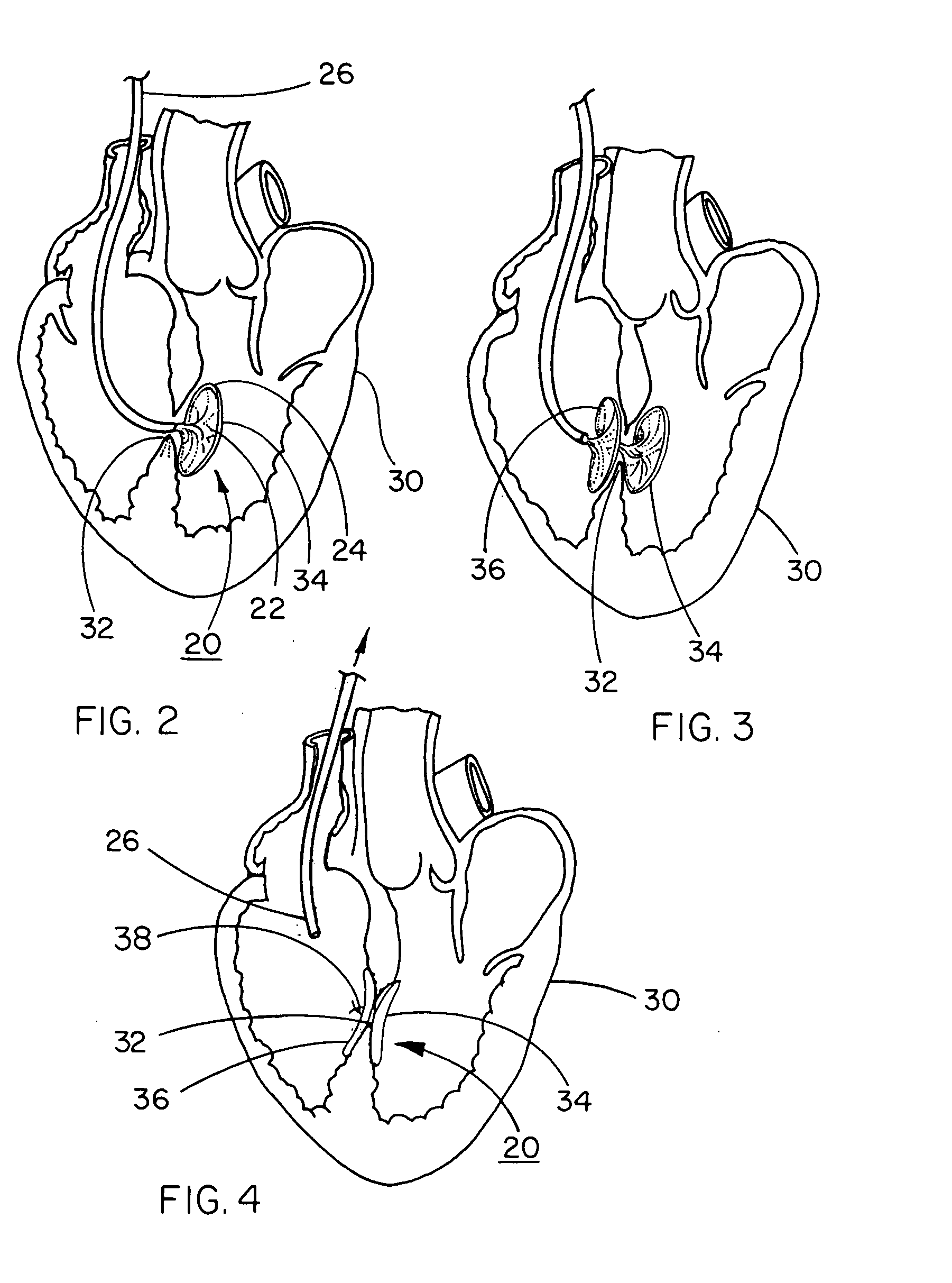
[0061] A HELEX™ Septal Occluder (SO) is acquired from W. L. Gore & Associates, Inc., Flagstaff, Ariz. This device, illustrated in FIGS. 1 through 4, comprises a nitinol metal frame and a porous expanded PTFE sheet wrapped around the metal frame.
[0062] The entire SO is immersed in 100% isopropyl alcohol for 30 seconds. The SO is then transferred to a 2% PVA / DI Water solution for 30 minutes. The SO is rinsed in DI water for 10 minutes and then placed in a 2% glutaraldehyde / 1% hydrochloric acid-DI water solution for 15 minutes. The SO is then rinsed in DI water for 15 minutes and allowed to air dry.
[0063] This final treated SO wetted-out rapidly when exposed to an aqueous solution, the membrane becoming completely translucent within 5 seconds after submersion in a water bath.
example 2
Process for Coating Stent-Graft
[0064] A VIATORR™ Stent-Graft is acquired from W. L. Gore & Associates, Inc., Flagstaff, Ariz. This device, designed for establishing a shunt through a patient's liver in a transjugular intrahepatic portacaval shunt (T.I.P.S.) procedure, comprises a nitinol metal stent-element that is partially covered with a tubular expanded PTFE graft element.
[0065] The stent-graft is placed in 100% IPA for 30 seconds and then immediately transferred into a 2% PVA / DI Water solution for 20 minutes. The stent-graft is then transferred into a DI water rinse for 15 minutes. The stent-graft is then placed in the 2% glutaraldehyde / 1% hydrochloric acid-DI water solution for 15 minutes. The stent-graft is then transferred into a final DI rinse for 15 minutes.
[0066] The final stent-graft device wet out rapidly when exposed to DI water, becoming completely translucent within 5 seconds after submersion in the water.
example 3
Process for Coating Embolic Filter
[0067] The filtering membrane was made by laser perforating one layer of a thin (total thickness about 0.0005 cm (0.0002 in)) polytetrafluoroethylene (PTFE) membrane from W. L. Gore & Associates, Elkton, Md. A hole pattern of uniform size and spacing was created. The perforated membrane was then folded on itself and heat-sealed using a soldering iron to create a conical shape. The conical flat pattern was then trimmed with scissors, inverted, and mounted on a tapered mandrel.
[0068] The conical filter membrane was attached to a nitinol metal frame using a fluorinated ethylene propylene (FEP) powder coated adhesive (FEP 5101, available from E. I duPont de Nemours & Co., Wilmington, Del.) and localized heat application.
[0069] Following embolic filter construction, the embolic filter was placed in 100% IPA for 30 seconds. The device was then immediately transferred into a 2% PVA / DI Water solution for 20 minutes. Then the device was transferred into a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| frequency | aaaaa | aaaaa |
| velocity | aaaaa | aaaaa |
| velocity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com