Composition for delivery of hematopoietic growth factor
a technology composition, which is applied in the direction of powder delivery, drug composition, peptide, etc., can solve the problems of multiple daily injections, fever and mild-to-moderate bone pain, and saw-toothlike effect of plasma drug levels, so as to increase the plasma half-life improve the activity of hematopoietic growth factor, and reduce the number of administrations.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Formulation of G-CSF with Pluronic™ F127
[0077] In one preferred embodiment of the present invention, the hematopoietic growth factor is G-CSF, and the delivery composition of the present invention provides a delivery system for the sustained administration of G-CSF to a human or animal host. A preferred first biocompatible polymer in this situation is a POE-POP block copolymer with reverse-thermal gelation properties.
[0078] As a specific formulation example, G-CSF can be formulated with Pluronic™ F127 (poloxamer 407), with and without hydroxypropylmethylcellulose (HPMC). Dry powder forms of Pluronic™ F 127 and HPMC are weighed, mixed together, and then reconstituted in water or physiological buffer to achieve the drug delivery matrix containing, upon addition of G-CSF, the desired concentrations of each component. More specifically, the concentration of Pluronic™ F127 is one that will achieve a final concentration (e.g., 5-30 weight %) at which it forms a semi-solid gel, along wit...
example 2
Administration of G-CCF with Pluronic™ 127
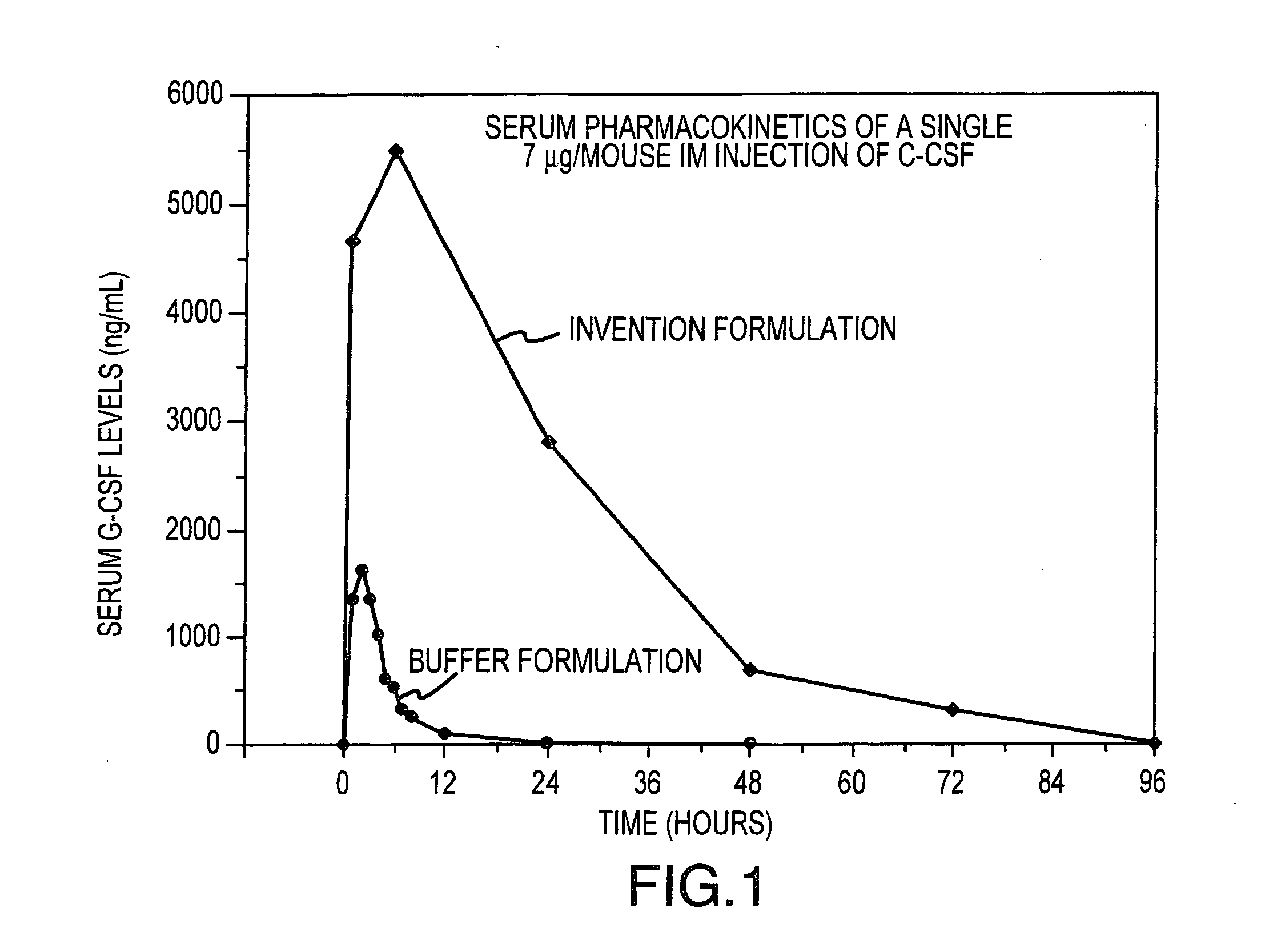
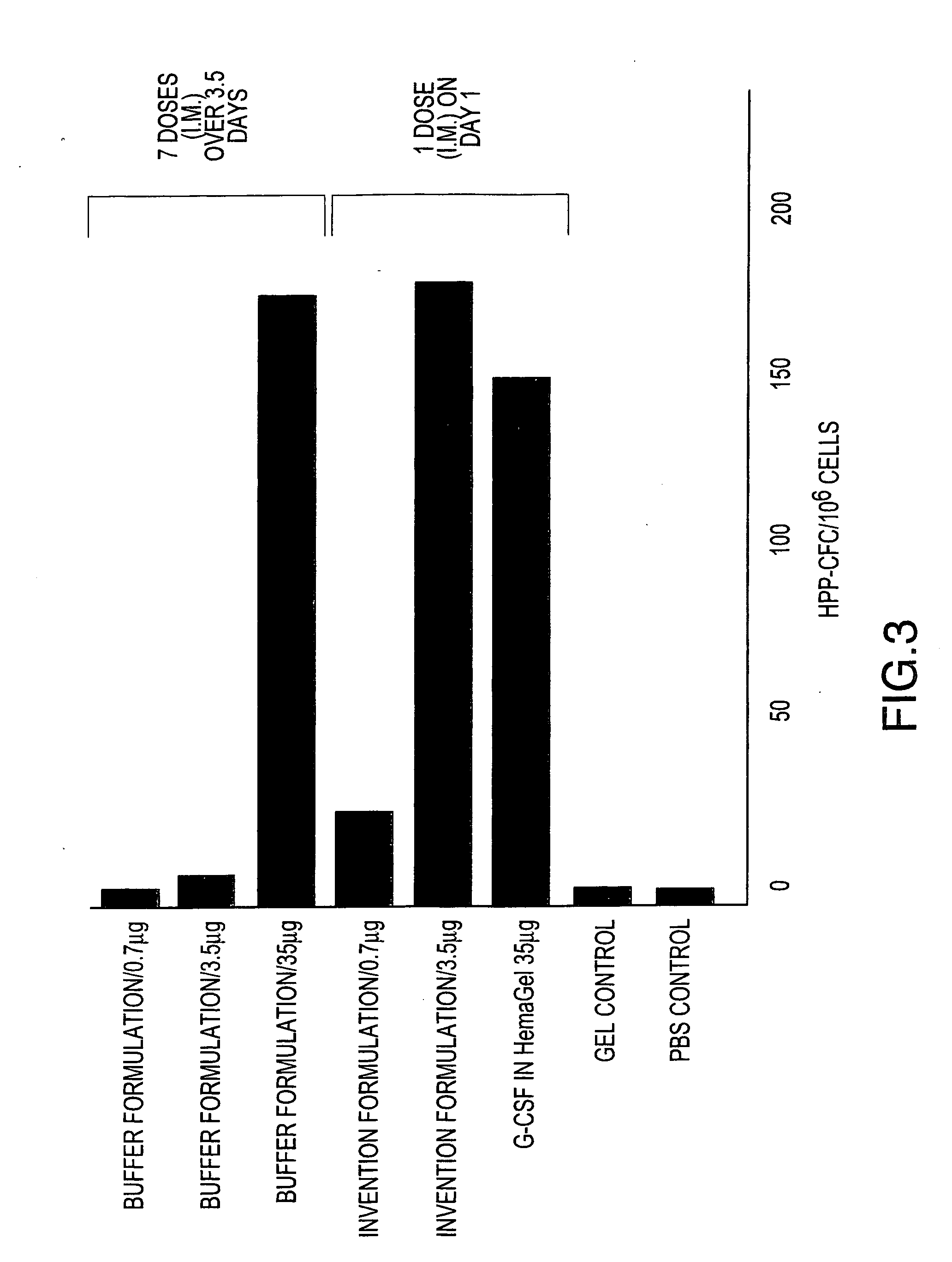
[0081] Formulations including G-CSF, Pluronic™ 127, with and without HPMC, are prepared and administered to groups of Balb / c mice to determine a) the effect of formulating G-CSF in a Pluronic™ 127 and HPMC (Invention Formulation) delivery matrix on the pharmacokinetic profile of G-CSF compared to conventionally (Buffer Formulation) formulated G-CSF and b) the effects of the Invention Formulation on hematopoietic activity compared to conventionally formulated G-CSF. The formulations are administered to mice intramuscularly (i.m.), as a single dose for pharmacokinetic analysis and as either single (for Invention Formulation) or multiple (for Buffer Formulation) doses for hematopoeitic acitivity. The compositions of the formulations are shown in Table 1.
TABLE 1Pluronic ™G-CSFHPMC (%GroupF127 (% w / w)(μg / mL)w / w)Vehicle control,000bufferVehicle control, gel1700.1 to 5G-CSF in buffer01 to 3000(BufferFormulation)G-CSF with177 to 1000.1 to 5Pluron...
example 3
Formulation of Flt3-L with Pluronic™ F127
[0090] In a preferred embodiment of the present invention, the hematopoietic growth factor is Flt3-L, and the pharmaceutical composition of the present invention provides a delivery system for the sustained administration of the Flt3-L to a human or animal. A preferred first biocompatible polymer in this situation is a POE-POP block copolymer with reverse-thermal gelation properties.
[0091] As a specific formulation example, Flt3-L can be formulated with Pluronic™ F127 (poloxamer 407), with or without hydroxypropylmethylcellulose (HPMC). Pluronic™ F127 is initially formulated in water or physiological buffer at concentrations (e.g., 5-30%) at which it forms a semi-solid gel, along with the addition of HPMC, at body temperature (37° C.). HPMC may then be added to the Pluronic™ F127 formulation at concentrations necessary to modulate the physicochemical properties of the Pluronic F127. (e.g., final concentrations of HPMC 1-5%). Alternatively, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com