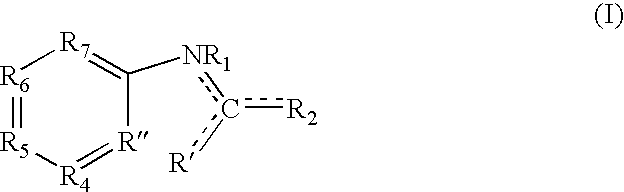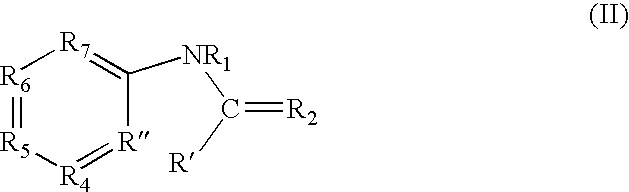Inhibitors of melanocyte tyrosinase as topical skin lighteners
a technology of melanocyte tyrosinase and topical skin lighteners, which is applied in the field of inhibitors of melanocyte tyrosinase, can solve the problems of hyperpigmentation lesions or skin regions, compound is not an effective inhibitor of mammalian enzymes, and is difficult to achieve in a large proportion of subjects, so as to prevent oxidative decomposition of product formulations and protect skin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Benzoimidazolethiols
[0385] A first class of compounds based upon the template compound benzimidazolethiol (lower left structure) were tested for tyrosinase inhibition, cell culture pigment inhibition, and toxicity, by methods described in Curto, E. V., et al. (1999) [25]. Results of the tests are given in Table 1.
TABLE 1ID #R1R2R3R5R6REPTελmax138NHSHNHHC0.25——14300300140NHSHNCH3HC0.122.4>10006300305084NHSHNOCH3HC0.071.6>100010000310040SSHNHHC8——091SSHNHOCH2CH3C>1000>1000>1000205NH═SN(CO)CH3HHC0.58.335098NH═SeNHHHC0.814132135NH═SNHHHN4256>1000
*Inhibition [μM] as measured in three assays. Here “E” is the concentration of compound that produces 50% pigment inhibition in the cell-free mammalian-enzyme assay system. “P” is for the concentration of compound that produces 50% inhibition in the mammalian-melanocyte-culture pigment assay system. “T” is the concentration of compound that kills 50% of cells in the
# mammalian-melanocyte-culture toxicity assay system. The compound extinction...
example 2
Thiophenols
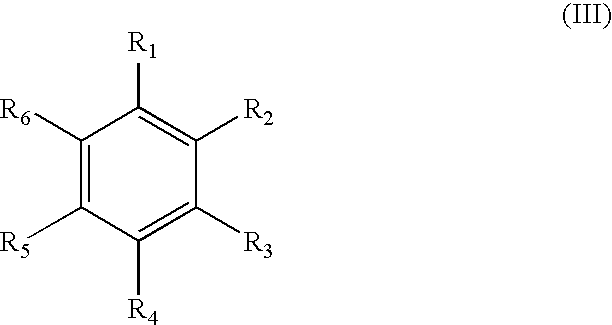
[0386] A second class of compounds based upon the template compound benzenethiol were tested for tyrosinase inhibition, cell culture pigment inhibition, and toxicity, by methods described in Curto, E. V., et al. (1999) [25]. Results of the tests are given in Table 2.
TABLE 2ID #R1R2R3R4R5EPTελmax099HHOCH3HH53852023000265102HHHSCH3H0.241151262300280083HHHNH(CO)CH3H19825424700265103HHOCH3OCH3H88>10004300250093HOCH3HHOCH35002002002700305148(CO)CH3HHNH(CO)CH3H500301253300255
*Inhibition [μM] as measured in three assays. Here “E” is the concentration of compound that produces 50% pigment inhibition in the cell-free mammalian-enzyme assay system. “P” is for the concentration of compound that produces 50% inhibition in the mammalian-melanocyte-culture pigment assay system. “T” is the concentration of compound that kills 50% of cells
# in the mammalian-melanocyte-culture toxicity assay system. The compound extinction coefficient is ε [OD / M × cm] at the wavelength of maximum abso...
example 3
Phenylthioureas
[0387] A third class of compounds based upon the template compound phenylthiourea (lower left structure) were tested for tyrosinase inhibition, cell culture pigment inhibition, and toxicity, by methods described in Curto, E. V., et al. (1999) [25]. Results of the tests are given in Table 3.
TABLE 3ID #R2R3R4R5REPTελmax033HHHHNH2212>1000181OCH3HHHNH2>1000>1000105HFHHNH21.521.78>100011000255104HOHHHNH248>1000131HCH3HHNH20.822.28>1000053HHOCH3HNH283060049HHNH(CS)NH2HNH24250>1000101HCH3HCH3NH2250125>1000054HHHHCH31616>1000
*Inhibition [μM] as measured in three assays. Here “E” is the concentration of compound that produces 50% pigment inhibition in the cell-free mammalian-enzyme assay system. “P” is for the concentration of compound that produces 50% inhibition in the mammalian-melanocyte-culture pigment assay system. “T” is the concentration of compound that kills 50% of cells in the
# mammalian-melanocyte-culture toxicity assay system. The compound extinction coefficie...
PUM
| Property | Measurement | Unit |
|---|---|---|
| color | aaaaa | aaaaa |
| wavelengths | aaaaa | aaaaa |
| hue | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com