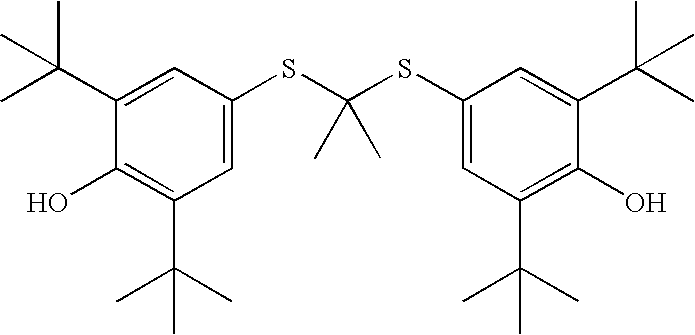
Thioketals and thioethers for inhibiting the expression of VCAM-1
a technology of thioketals and thioethers, which is applied in the direction of sugar derivatives, aminosugars, biocides, etc., can solve the problems of limited expression of icam-1 and vcam-1 in normal cardiac tissu
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Thioketal Synthetic Methods
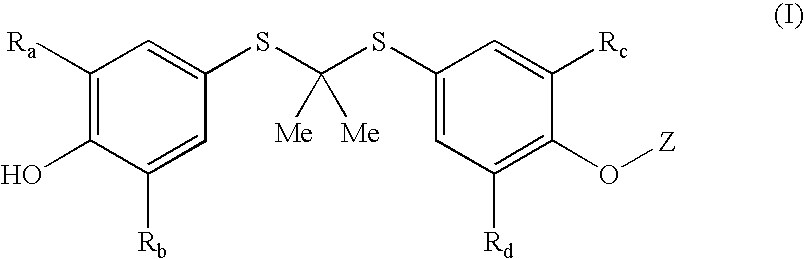
[0144] The thioketal compounds of formula (I) wherein Z forms an ether group can be prepared by known procedures and techniques, or routine modifications thereof. General procedures for preparing compounds of formula (I) wherein Z forms an ether group are set forth in General Procedures A and B, wherein all substituents, unless otherwise indicated, are previously defined.
[0145] Compounds of the present invention can be readily prepared by someone skilled in the art of organic synthesis using a standard ether coupling procedure known as the Mitsunobu reaction (Hughes, D. L. Org. Prep. Proced. Int., 28, 127-64 (1996)). For example, to a solution of probucol in a suitable aprotic solvent such as tetrahydrofuran (THF) are added triphenylphosphine, diethyl azodicarboxylate, and an appropriate alcohol moiety optionally containing the additional substituents described above for Z. The resultant mixture is stirred under nitrogen at reflux for 2 to 8 hours and t...
example 4
Thioether Synthetic Methods
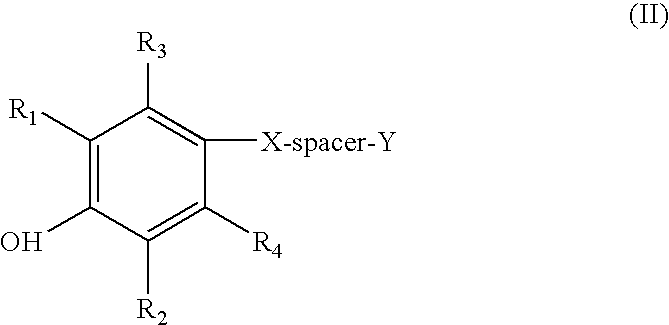
[0158] The thioethers of formula (II) can be prepared by utilizing known procedures and techniques, or routine modifications thereof. See, for example, the synthetic methods disclosed in PCT / US98 / 09781 published as WO 98 / 51662. A general synthetic method for preparing compounds of formula (II) is set forth in General Procedure C, wherein all substituents, unless otherwise indicated, are previously defined.
[0159] General Procedure C
[0160] The synthesis of the starting thiol, 4-mercapto-2,6-di-t-butylphenol, is described in the literature (U.S. Pat. No. 3,129,262 to Laufer, incorporated herein by reference in its entirety). The starting alkyl halides are commercially available or made from commercially available starting materials by methods known to one of ordinary skill in the art.
[0161] A quantity of the 4-mercapto-2,6-di-t-butylphenol is dissolved in ethanol to make a 0.5 M solution and treated with 1.2 equivalents of sodium hydroxide (5 N aqueous so...
example 6
2,6-Di-tert-butyl-[4′-(N,N-diethylaminocarbonyl)benzyl]thiophenol
[0170] Same as Example 3, but substitute diethylamine for dimethylamine.
[0171] The following examples illustrate the use of thioketals and thioethers according to the present invention. These examples are illustrative only and are not intended to limit the scope of the invention in any way.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com