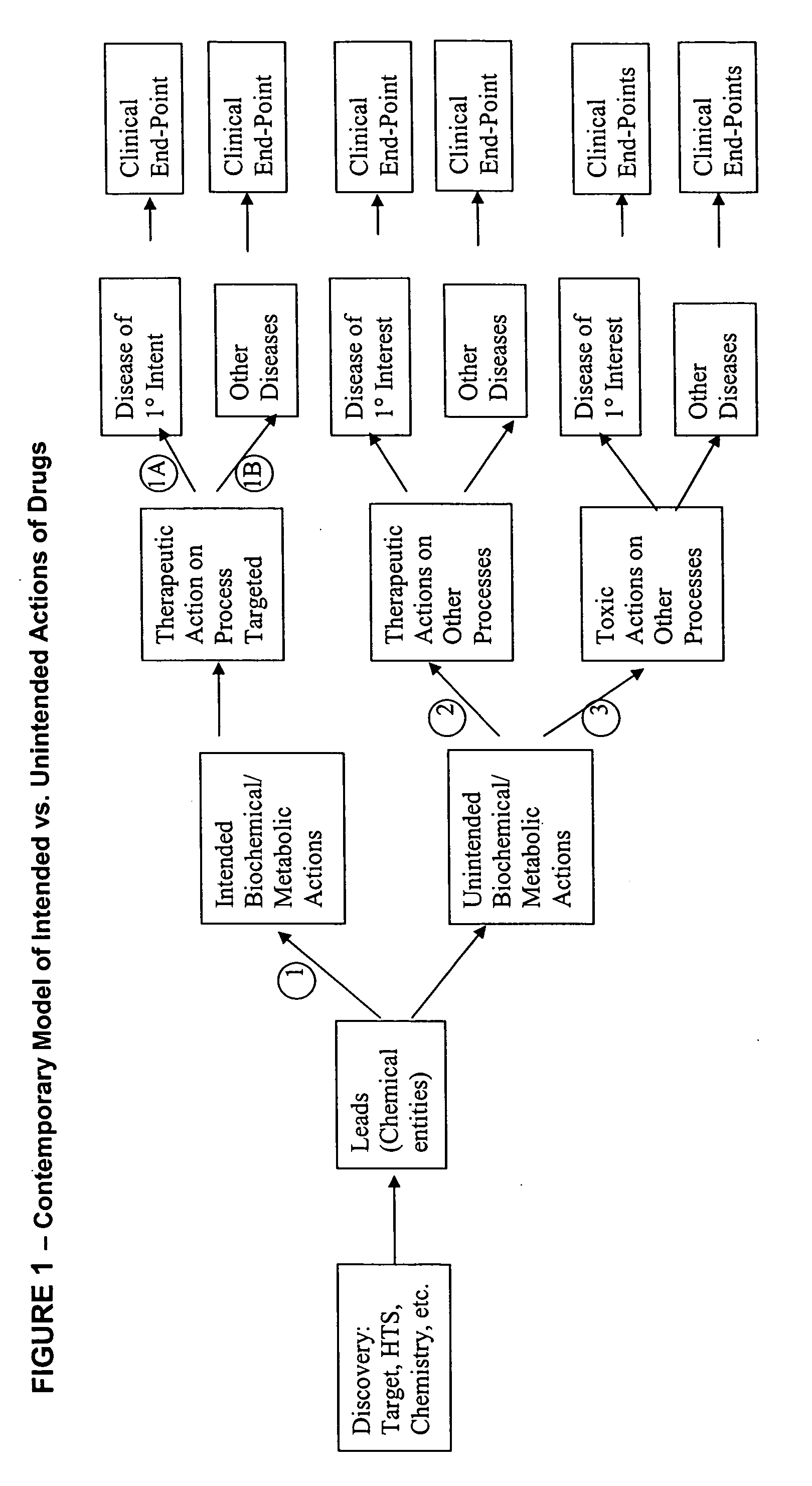
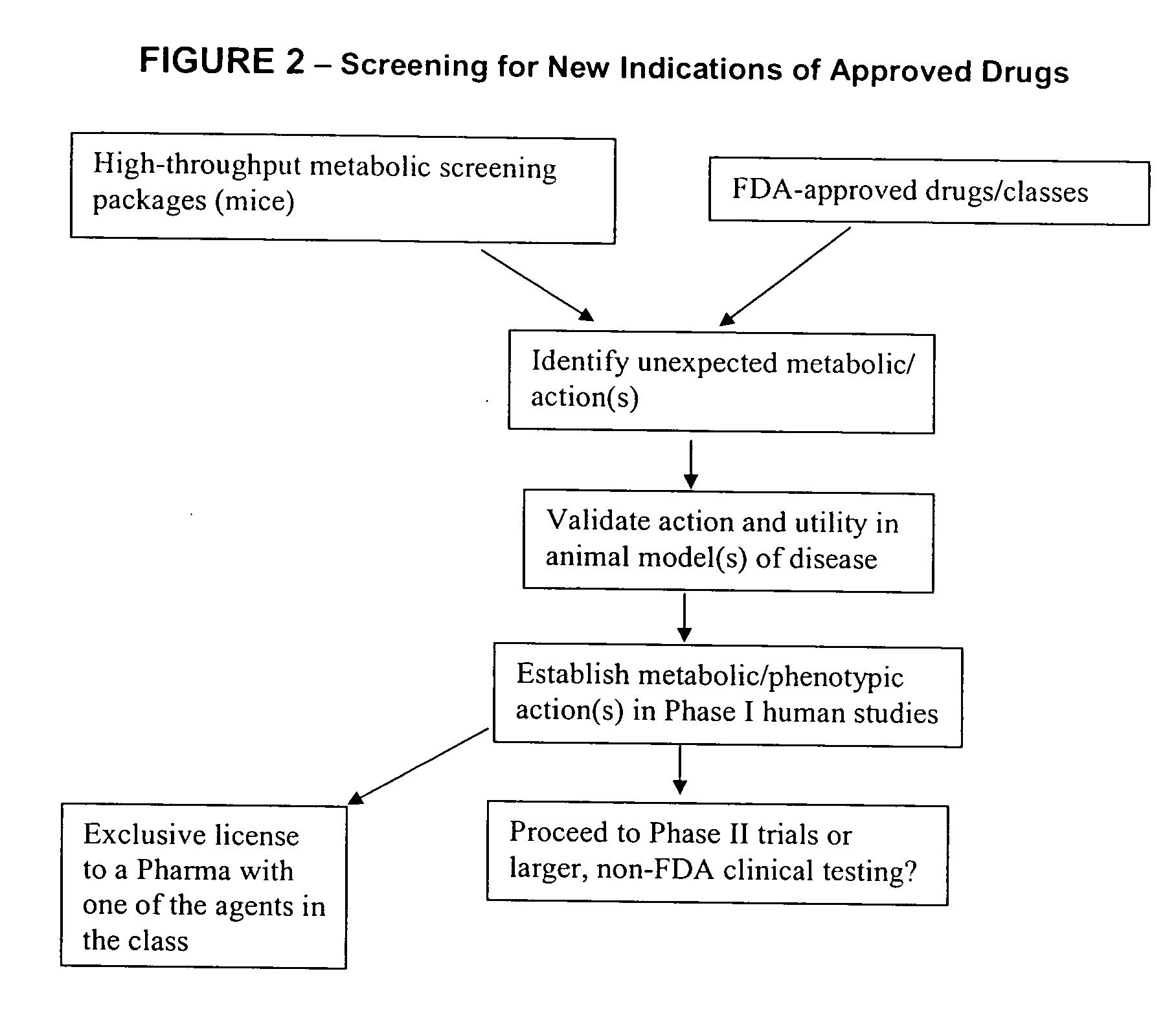
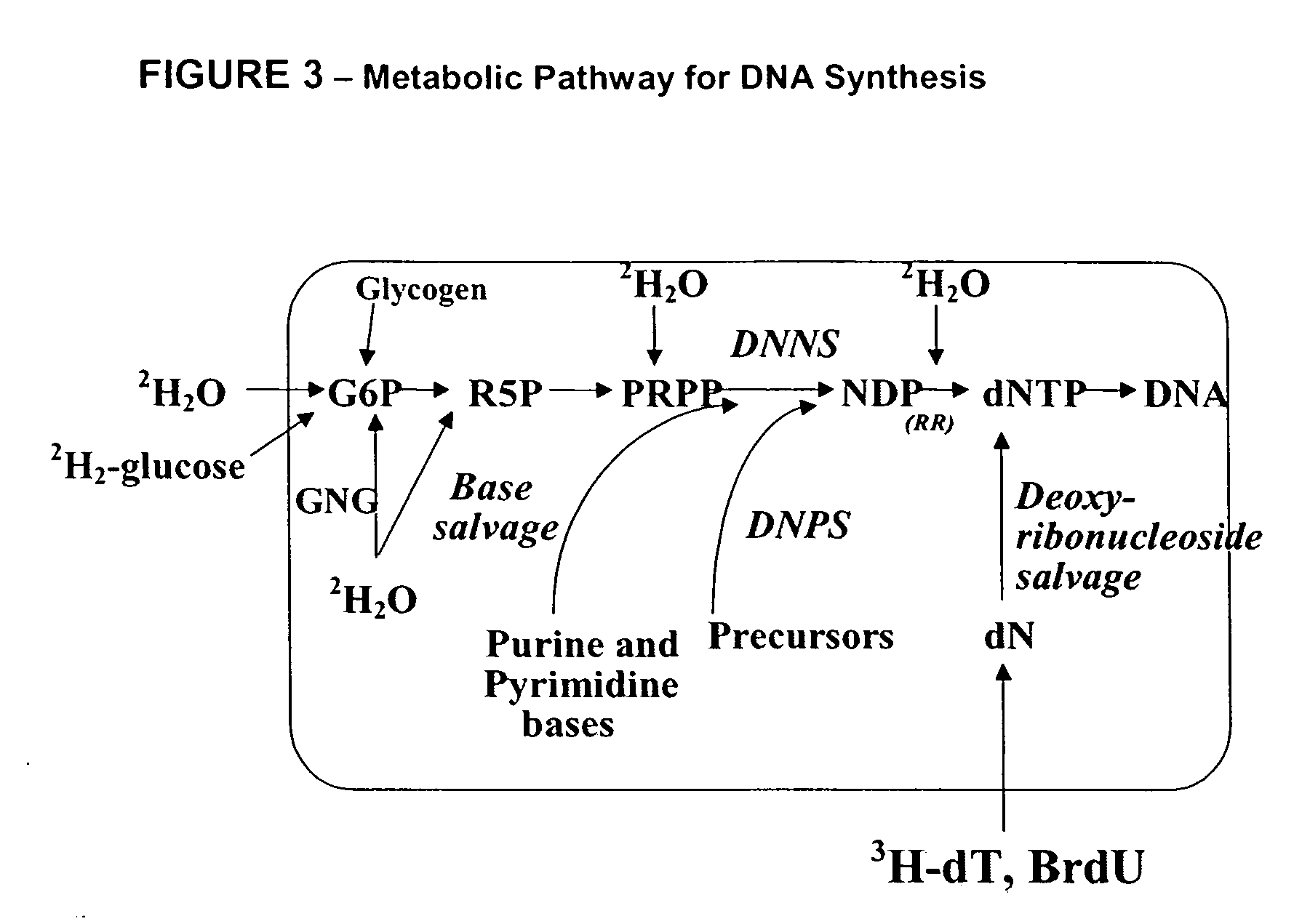
Method for high-throughput screening of compounds and combinations of compounds for discovery and quantification of actions, particularly unanticipated therapeutic or toxic actions, in biological systems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Measurement of Triglyceride (TG) Synthesis (Lipogenesis) and Breakdown (Lipolysis) in Rats after Exposure to Compounds
[0239] To assess whether a compound inhibits lipogenesis (and therefore, a candidate drug for treating obesity or other metabolic disorders) Sprague-Dawley rats (200-300 g Simonsen Labs, Gilroy, Calif.) are either exposed to a compound or left unexposed (i.e., controls). Rats are administered compound or vehicle via gavage. One or several compounds may be administered. For example, thousands of compounds may be initially screened, pooled, rescreened, subpooled, etc., to screen for one or more active compounds. An initial priming dose of 99.8% 2H2O is given via intraperitoneal injection to achieve ca. 2.5% body water enrichment (assuming 60% body weight as water) followed by administration of 4% 2H2O in drinking water for up to 12 weeks.
[0240] Adipose tissue samples are placed in dual glass tissue grinders (e.g., Kontes tissue grinders, Kimble Kontes, Vineland, N.J....
example 2
Measurement of DNA Synthesis and Breakdown in Rats after Exposure to Compounds
[0250] DNA synthesis is a biomarker for cell proliferation. In some settings it may be desirable to stimulate cell proliferation (e.g., to stimulate wound healing) while in other settings it may be desirable to inhibit cell proliferation (e.g., cancer).
[0251] Rats are administered 2H2O as discussed in Example 1, supra.
[0252] Rats are either administered compounds (or combinations of compounds or mixtures of compounds) or vehicle (controls) as discussed in Example 1, supra.
[0253] DNA is then isolated from the tissue or cell of interest using a Qiagen kit (Qiagen, Valencia, Calif.), following the manufacturer's protocol.
[0254] Isotope enrichment is then analyzed and flux rates calculated as described, supra. DNA synthesis is then determined as described, supra, (and in U.S. Pat. No. 5,910,403, incorporated by reference). Compound-exposed animals are then compared to unexposed animals to determine if the...
example 3
Measurement of Neurogenesis in Rat Hippocampal Neuroprogenitor Cells after Exposure to Compounds
[0255] Compounds (or combinations of compounds or mixtures of compounds) are tested on rats to determine whether one or more may have effects on neurogenesis. Compounds with neurogenic potential may find use in treating spinal cord injury, Parkinson's disease, Huntington's disease and other neurodegenerative disorders. Rats are divided into exposed and control groups and administered labeled water as in Example 1, supra. After exposure to compound or combinations of compounds or mixtures of compounds (or vehicle if control rat), by gavage, intrathecal, or intracranial administration (route of administration is dependent on the chemistry of the compound or combination of compounds or mixture of compounds, as is well known in the art) rats are deeply anesthetized with a mixture of ketamine, xylazine, and acepromazine. Rats are then decapitated and whole brains are removed.
[0256] For isola...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar concentration | aaaaa | aaaaa |
| natural abundance | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com