Method and cell composition for screening compounds for anti-inflammatory activity
a technology of anti-inflammatory activity and cell composition, which is applied in the field of method, cell composition and assay for screening compounds for anti-inflammatory activity in vitro, can solve the problems of eventual mortality of the host and morbid states
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of a pcCMV-PKR Vector and a PKR(−) Control Vector
[0148] cDNA encoding the full-length human PKR molecule (551 amino acids; Meurs, et al., 1990; GenBank Accession No. NM002759) was inserted into a eukaryotic expression vector, such that the PKR coding sequence was expressed under the control of the CMV promoter.
[0149] The vector contains various features suitable for PKR transcription, including: i) a promoter sequence from the immediate early gene of the human CMV for high level mRNA expression; ii) a polyadenylation signal and transcription termination sequences from the β-globin gene to enhance RNA stability; iii) an ampicillin resistance gene and ColE1 origin for selection and maintenance in E. coli.
[0150] A second vector contained i) an ampicillin resistance gene and a ColE1 origin for selection and maintenance in E. coli; ii) the G418 resistance marker (Neo) to allow for selection and identification of the plasmids after co-transfection into eukaryotic cells.
example 2
Preparation and Analysis of a PKR Over-producing Namalwa Cell Line Over-expressing Inflammatory Cytokines
[0151] Stable transfectants were obtained by electroporation of 4×106 exponentially growing Namalwa cells with 15 μg of the vector pCMV-PKR and 15 μg of the Neo containing second vector in DMEM / F12 (+10% FBS) using a Gene Pulser apparatus (BioRad) set at 800 μF, 300 V. Bulk populations of stable transformants were obtained by selection with 2 mg / ml geneticin (Gibco-BRL) for 3-4 weeks. Clonal lines were subsequently obtained by limiting dilution cloning.
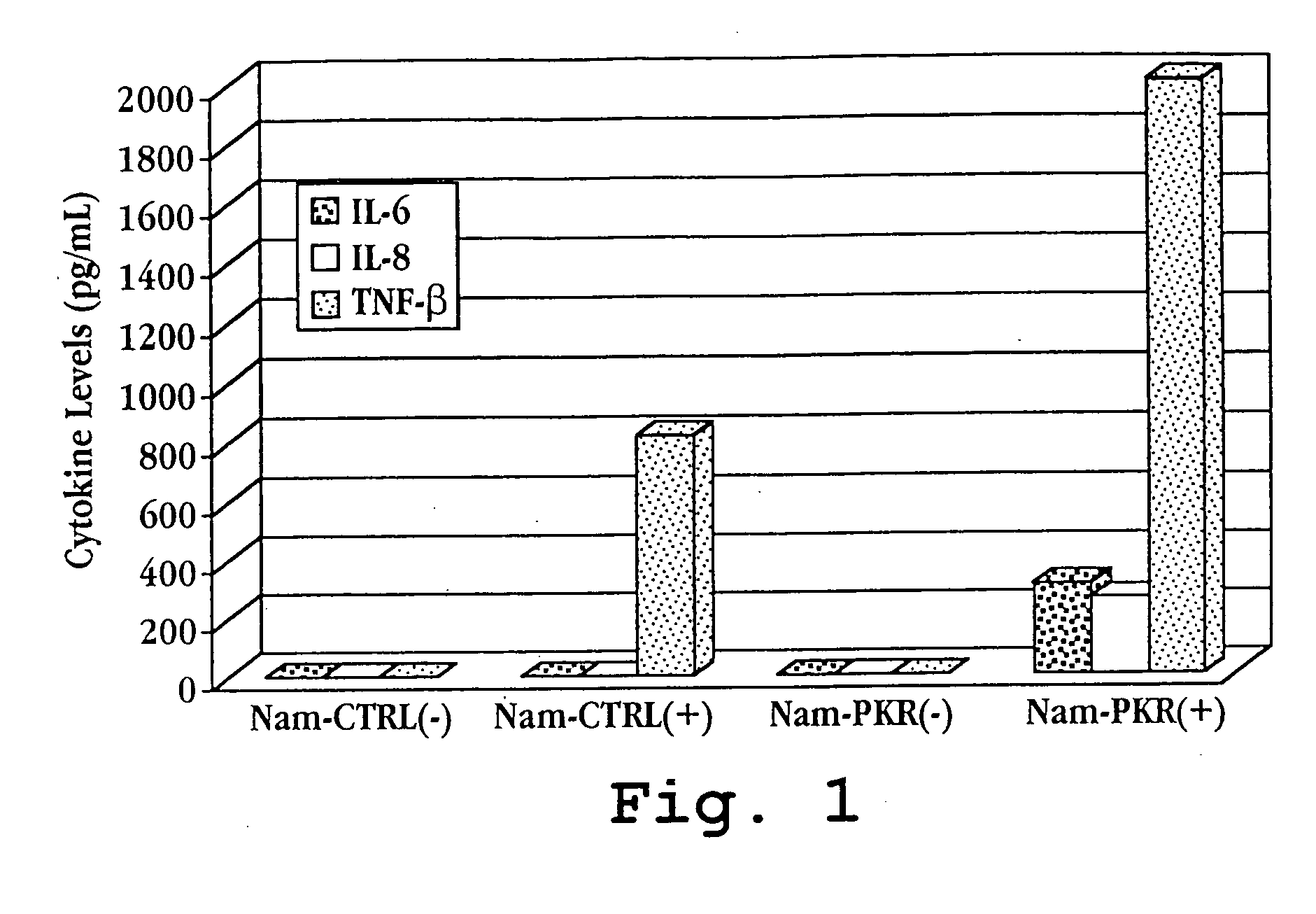
[0152] A. Increased Inflammatory Cytokine Expression Following Induction
[0153] The level of PKR in the parental and PKR-transfected Namalwa cells were analyzed by Western blot and PKR activity assay (Zamanian-Daroush, et al., 1999). PKR expression was found to have increased approximately sixteen-fold in the PKR-transfectants relative to the parental Namalwa cells.
[0154] PKR-transfected Namalwa cells (Nam-PKR) and parental Nama...
example 3
Suppression of PKR-mediated Inflammatory Cytokine Production by a PKR-Specific Inhibitor and Potential Anti-inflammatory Drug
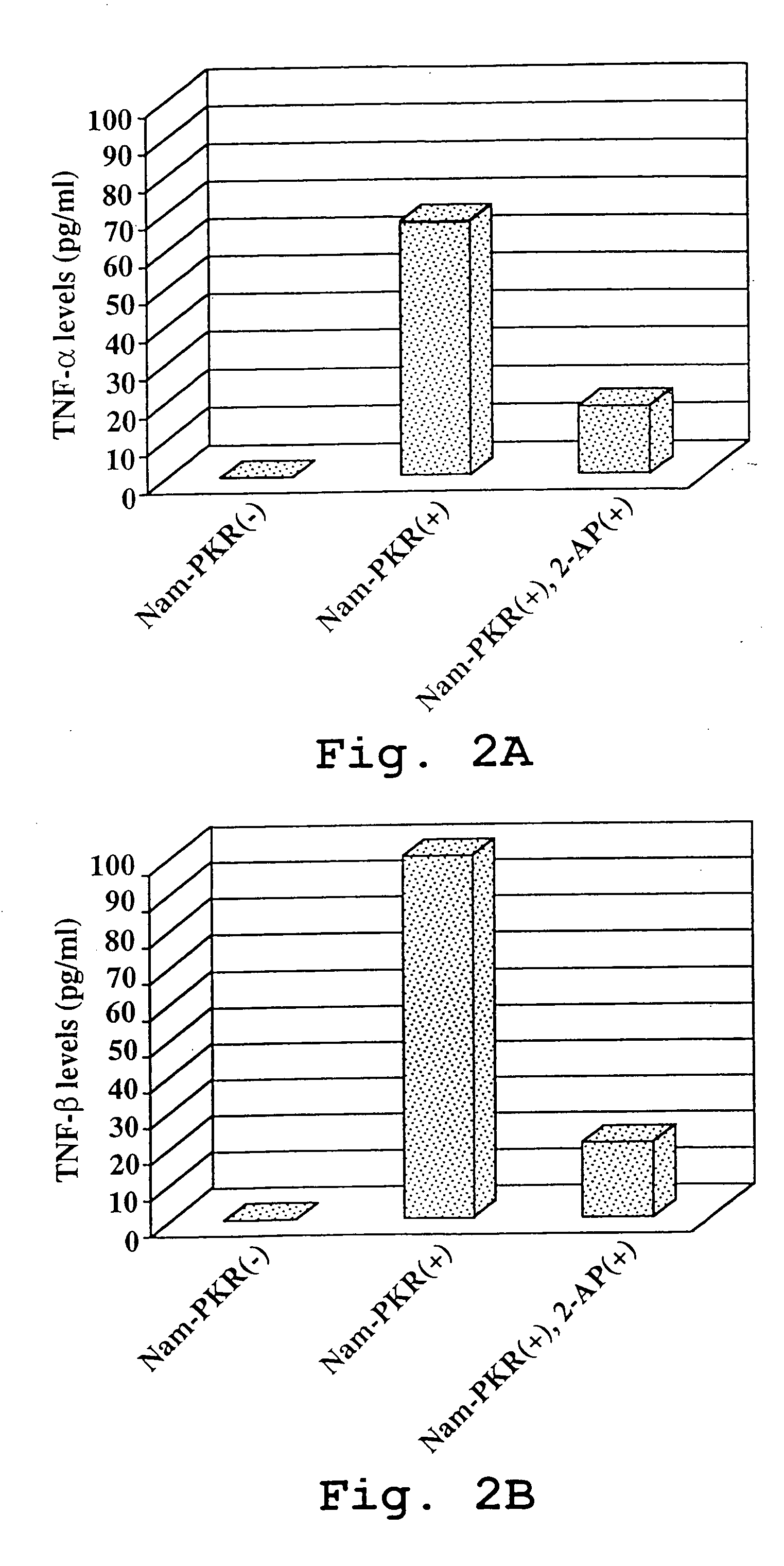
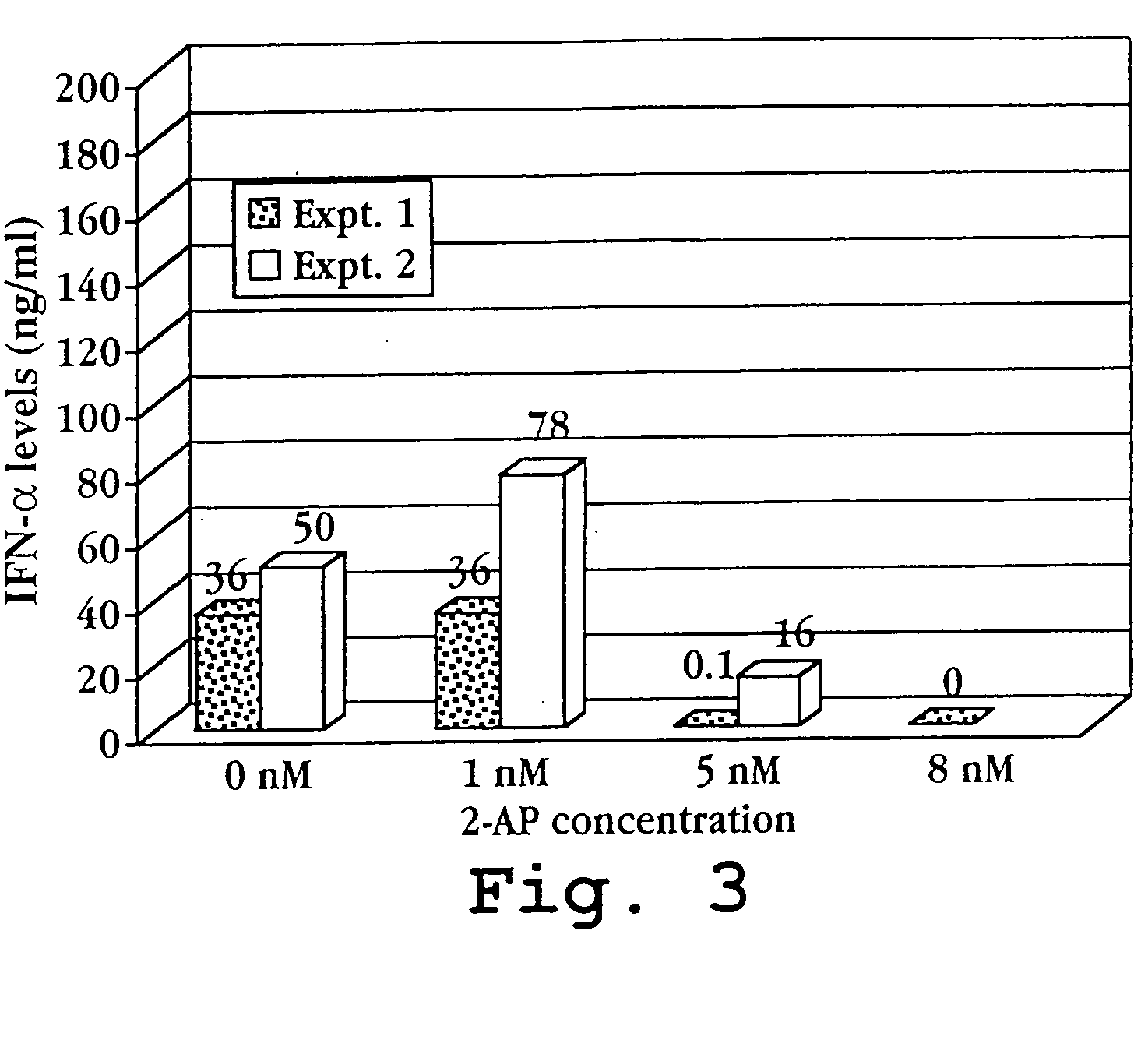
[0156] A. Suppression of PKR-mediated Inflammatory Cytokine Production by 2 Aminopurine
[0157] PKR-transfected Namalwa cells (Nam-PKR) were cultured at 2.5×105 cells / ml in DMEM / F12 medium supplemented with 10% FBS. The cells were treated with 20 mM PMA (priming) for 20 hr followed by treatment with 200 μg / ml poly I:C (induction) for 3 days in the presence of 1 mM 2-aminopurine (2-AP) or in the absence of 2-AP. One set of cells was left untreated (non-induced controls). Following treatment, the culture supernatants were collected and analyzed for TNF-alpha or for TNF-beta by ELISA according to the procedure provided by the supplier of the ELISA kits (R&D Systems). The data for cytokine induction is shown in FIGS. 2A and 2B, which illustrates that the PKR-overexpressing Namalwa cell line produces a high level of proinflammatory cytokine (TNF-alpha) under induct...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Anti-inflammatory | aaaaa | aaaaa |
| Fluorescence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com