Protein purification
a technology of protein purification and purification chamber, applied in the field of protein purification, can solve the problems of difficult removal of subcellular fragments, formidable challenge of human use as a human therapeutic,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Intermediate Wash Solutions
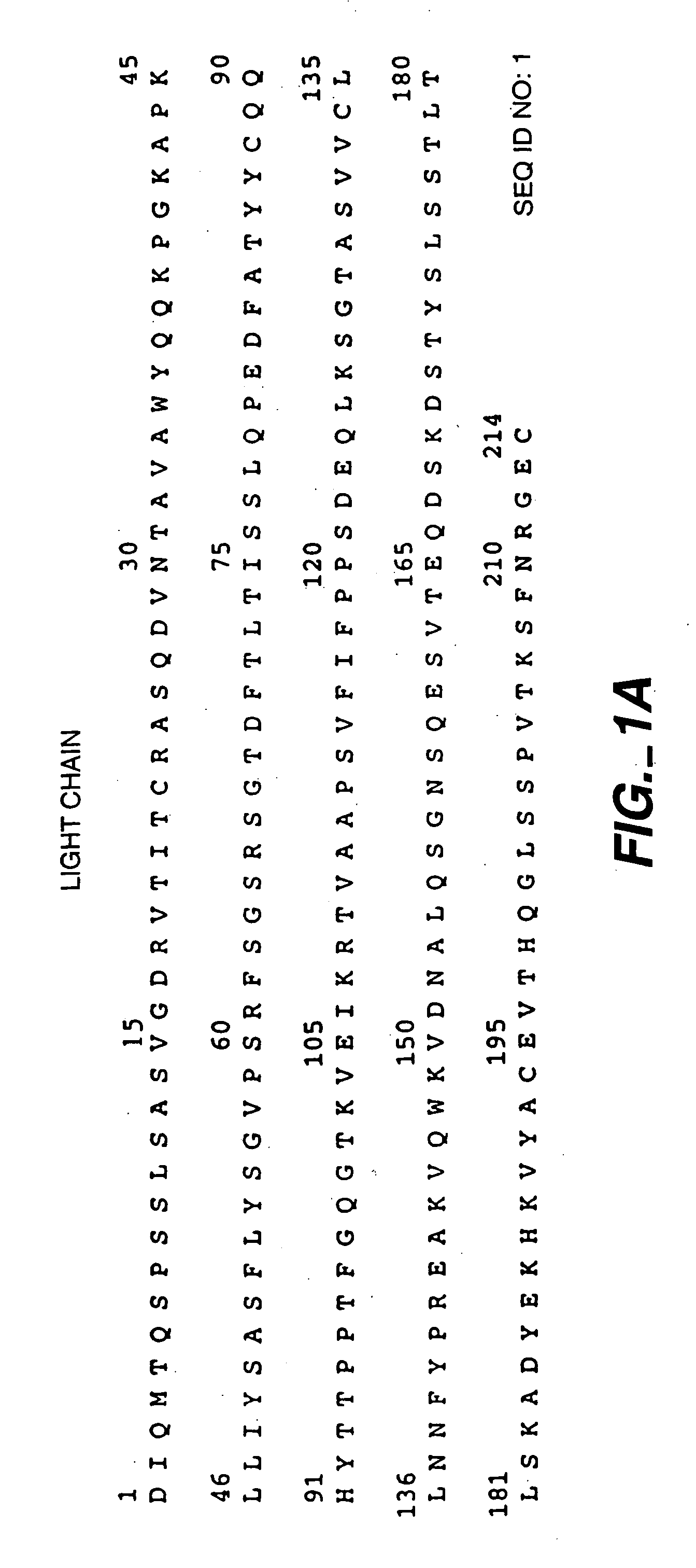
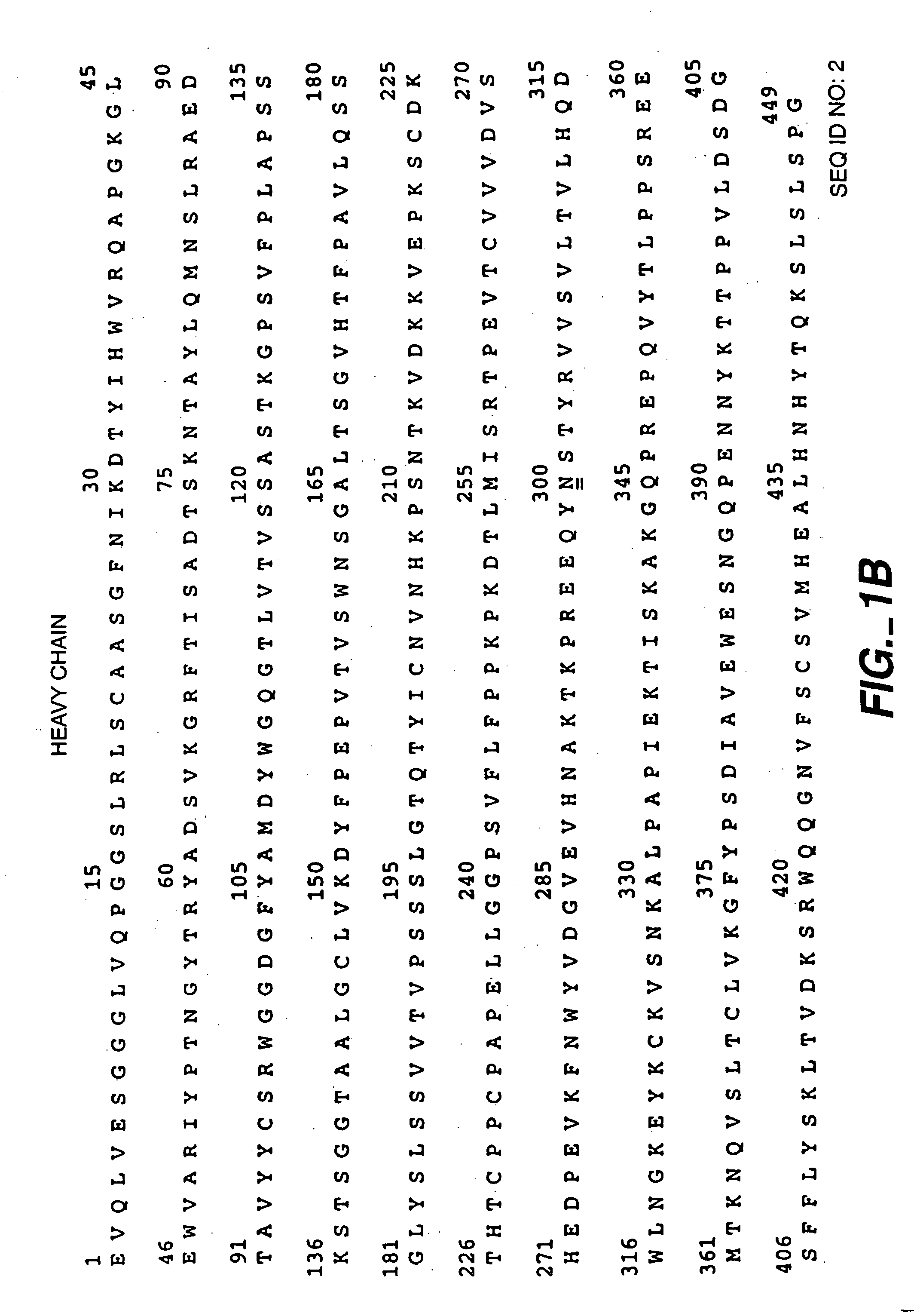
[0126] The anti-HER2 antibody Trastuzumab (FIGS. 1A-B; HERCEPTIN®), anti-IgE antibody (E26; U.S. Pat. No. 5,994,511, Lowman et al.) and humanized anti-CD11a antibody (XANELIM®; U.S. Pat. No. 6,037,454) were recombinantly produced in Chinese Hamster Ovary (CHO) cells and purified by Protein A chromatography as a first chromatographic step to remove contaminating CHO proteins (CHOP). However, CHOP tends to binding non-specifically to ProSepA, the resin used for this step. ProSep A has the antibody-binding Protein A immoblized on glycerol-coated controlled-pore glass. Although the glycerol coat reduces non-specific binding, some CHOP still adheres to the resin's glass backbone. During the elution phase of the Protein A operation, any non-specifically bound CHOP will co-elute with the antibody, compromising the purity of the product pool. To remove this CHOP before the elution phase, U.S. Pat. Nos. 6,127,526 and 6,333,398 (Blank, G.) exemplify an intermediate...
example 2
Alteration of the Intermediate Wash Buffer
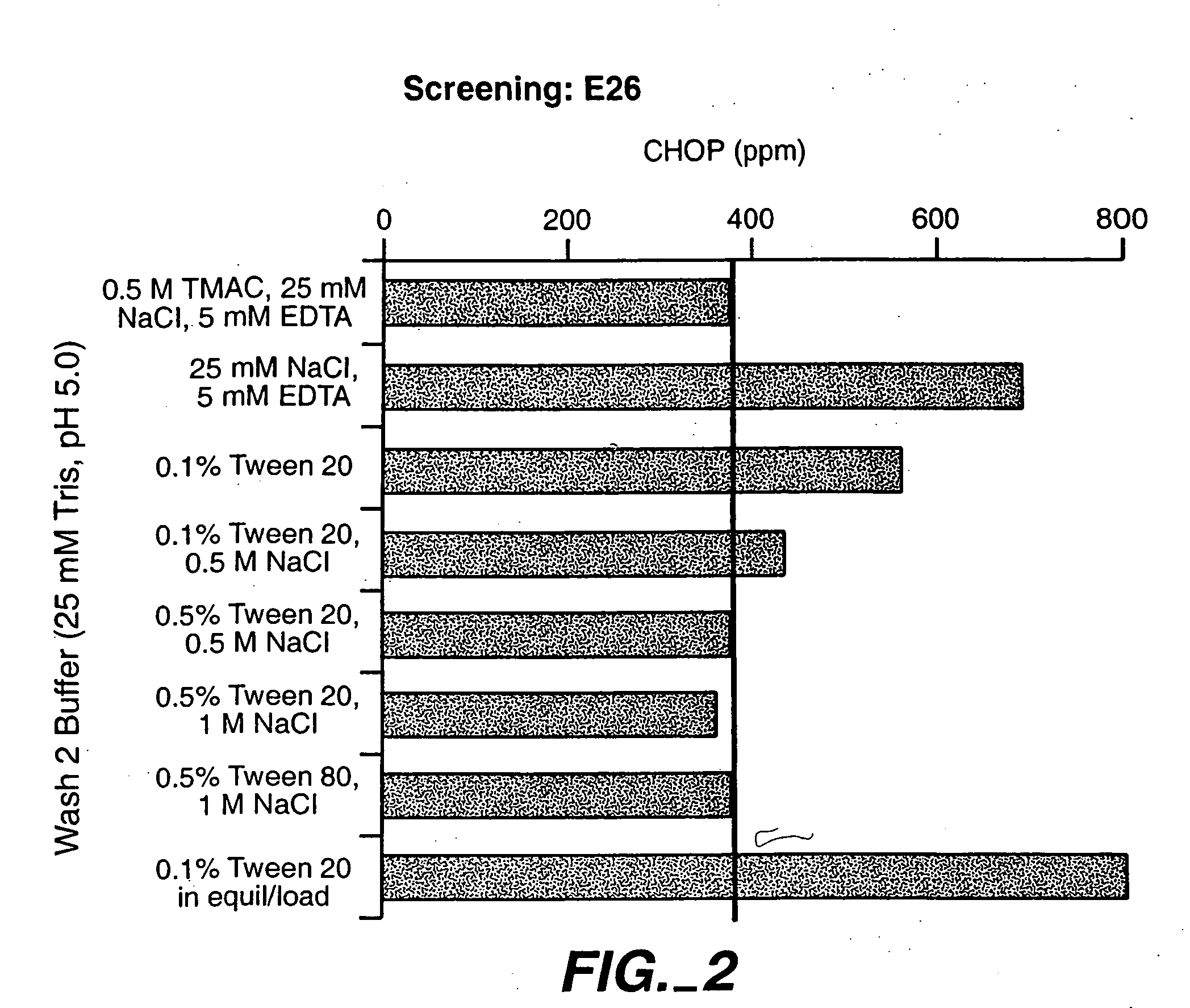
[0130] The effect of (a) salt type and concentration, (b) polysorbate concentration, and (c) pH of the intermediate wash buffer on CHOP removal was evaluated. The antibodies were the anti-IgE antibody E26, and the anti-HER2 antibody Trastuzumab.
[0131]FIG. 7 depicts the effect of salt type and concentration on CHOP removal. For E26, CHOP level in the elution pool was not significantly affected by concentration of citrate or sulphate in the wash solution. For Trastuzumab, CHOP level in the elution pool was affected by the concentration of salt in the intermediate wash buffer. Preferred concentrations of the salt were from about 0.3 to about 0.6M.
[0132] The effect of polysorbate concentration on CHOP level in the elution pool was also evaluated. As shown in FIG. 8, as the concentration of polysorbate in the intermediate wash buffer increased, the amount of CHOP contamination decreased. The preferred concentration of polysorbate is about 0.5%...
example 3
Downstream Performance
[0134] The downstream performance in terms of CHOP removal, yield, etc (SDS-PAGE, HPLC-IEC, size exclusion chromatography (SEC), and protein A leaching), were determined for E26 and Trastuzumab. The downstream purification steps were cation exchange chromatography (SP-Sepharose) and anion exchange chromatography (Q-Sepharose).
[0135] For E26, the intermediate wash buffers were: (a) 0.5% polysorbate 20 / 0.2M sodium citrate, (b) 20% hexylene glycol / 0.2M sodium citrate, and (c) 1M Tris acetate. The results are shown in the following Table.
TABLE 1E26 DownstreamCHOP (ppm)StepTMACPolys / SaltHex Gly / SaltTrisOAcEquilPro A320260230310600SP-Seph5040304090Q-Seph4Overall %7472677372Yield:
[0136] For Trastuzumab, the intermediate wash buffers were: (a) 0.5% polysorbate 20 / 0.5M sodium acetate, (b) 10% hexylene glycol / 0.5M sodium acetate, and (c) 1M Tris acetate. The results are summarized in the Tables below.
TABLE 2Trastuzumab DownstreamCHOP (ppm)StepTMACPolys / SaltHex Gly / ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com