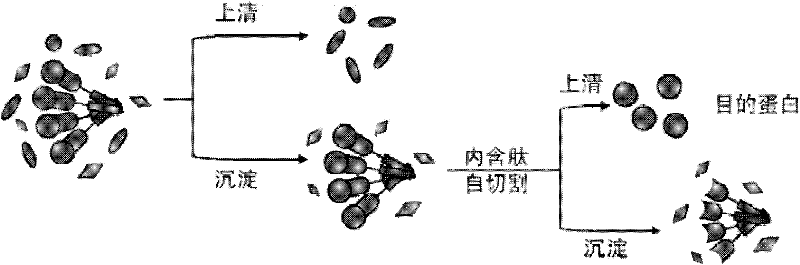
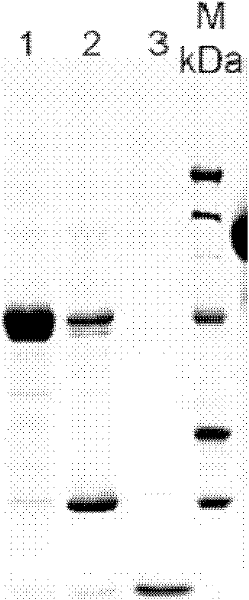
Method for purifying protein of enzyme aggregate based on self-aggregation short-peptide induction
A technology for protein purification and enzyme aggregation, applied in the direction of microorganism-based methods, biochemical equipment and methods, and the use of vectors to introduce foreign genetic materials, etc., can solve the problems of many operation steps and high costs, and achieve cost reduction and high economy , The effect of overcoming the bottleneck of industrial application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Expression and purification of a triplet fusion protein using wild-type Bacillus subtilis lipase A (LipA) as the target protein (fused at the N-terminus)
[0039] Strain: Escherichia coli BL21(DE3)
[0040] Vector: pET-30a(+)
[0041] Medium: LB / Kan r , each liter medium contains tryptone 10.0g, yeast extract powder 5.0g, NaCl 10.0g, if it is a solid medium, add agar 15.0g per liter medium, pH7.0, 50μg / ml kanamycin ( Kanamycine).
[0042] 1. Construction of triplet fusion protein expression vectors pET-30a(+)-LipA-Mxe-ELK16 and pET-30a(+)-LipA-Mxe-18A
[0043] The pET-30a(+)-LipA-ELK16 plasmid was extracted using the high-purity plasmid small extraction kit from Tiangen Company (ELK16 is fused to LipA at the C-terminus, and the plasmid structure is as follows Figure 6 shown. Its construction method is to select the pET-30a (+) plasmid of commercial plasmid Novogen Company, use the online tool DNAworks to design Linker and ELK16 nucleotide sequence, utili...
Embodiment 2
[0051] Example 2 Expression and purification of Aspergillus fumigatus Amadoriase II (AMA) as a triplet fusion protein of the target protein (fused at the N-terminus).
[0052] Strain: Escherichia coli BL21(DE3)
[0053] Vector: pET-30a(+)
[0054] Medium: LB / Kan r , each liter medium contains tryptone 10.0g, yeast extract powder 5.0g, NaCl 10.0g, if it is a solid medium, add agar 15.0g per liter medium, pH7.0, 50μg / ml kanamycin ( Kanamycine).
[0055] 1. Construction of triplet fusion protein expression vectors pET-30a(+)-AMA-Mxe-ELK16 and pET-30a(+)-AMA-Mxe-18A
[0056] The pET-30a(+)-LipA-Mxe-ELK16 plasmid was extracted using the high-purity plasmid mini-extraction kit from Tiangen Company (see Example 1 for the construction method, Figure 7 ), pET-30a(+)-LipA-Mxe-18A (see Example 1 for the construction method, Figure 7 ) and pET-30a(+)-AMA-ELK16 plasmid (ELK16 is fused with AMA at the C-terminus, the plasmid structure is as follows Figure 8 As shown, its construction...
Embodiment 3
[0061] Example 3 Expression of Bacillus pumilus xylosidase (XynB) as a triplet fusion protein (fused at the N-terminus) of the target protein and its purification
[0062] Strain: Escherichia coli BL21(DE3)
[0063] Vector: pET-30a(+)
[0064] Medium: LB / Kan r , each liter medium contains tryptone 10.0g, yeast extract powder 5.0g, NaCl 10.0g, if it is a solid medium, add agar 15.0g per liter medium, pH7.0, 50μg / ml kanamycin ( Kanamycine).
[0065] 1. Construction of triplet fusion protein expression vectors pET-30a(+)-XynB-Mxe-ELK16 and pET-30a(+)-XynB-Mxe-18A
[0066] The pET-30a(+)-LipA-Mxe-ELK16 plasmid was extracted using the high-purity plasmid mini-extraction kit from Tiangen Company (see Example 1 for the construction method, and the plasmid structure is as follows Figure 7 shown), pET-30a(+)-LipA-Mxe-18A (see Example 1 for the construction method, the plasmid structure is as follows Figure 7 shown) and pET-30a(+)-XynB-ELK16 plasmid (ELK16 is fused with XynB at t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com