Mucosal cytotoxic T lymphocyte responses
a technology of mucosal immune response and cytotoxic t lymphocytes, which is applied in the direction of antibody medical ingredients, dsdna viruses, peptide sources, etc., can solve the problems of inability prior investigations have failed to identify fundamental mechanisms linking immune responses to protection, and administration of antigens via parenteral routes (subcutaneously). , to achieve the effect of enhancing the ctl response, reducing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Comparison of Mucosal and Systemic-CTL Responses After Mucosal and Systemic Immunization
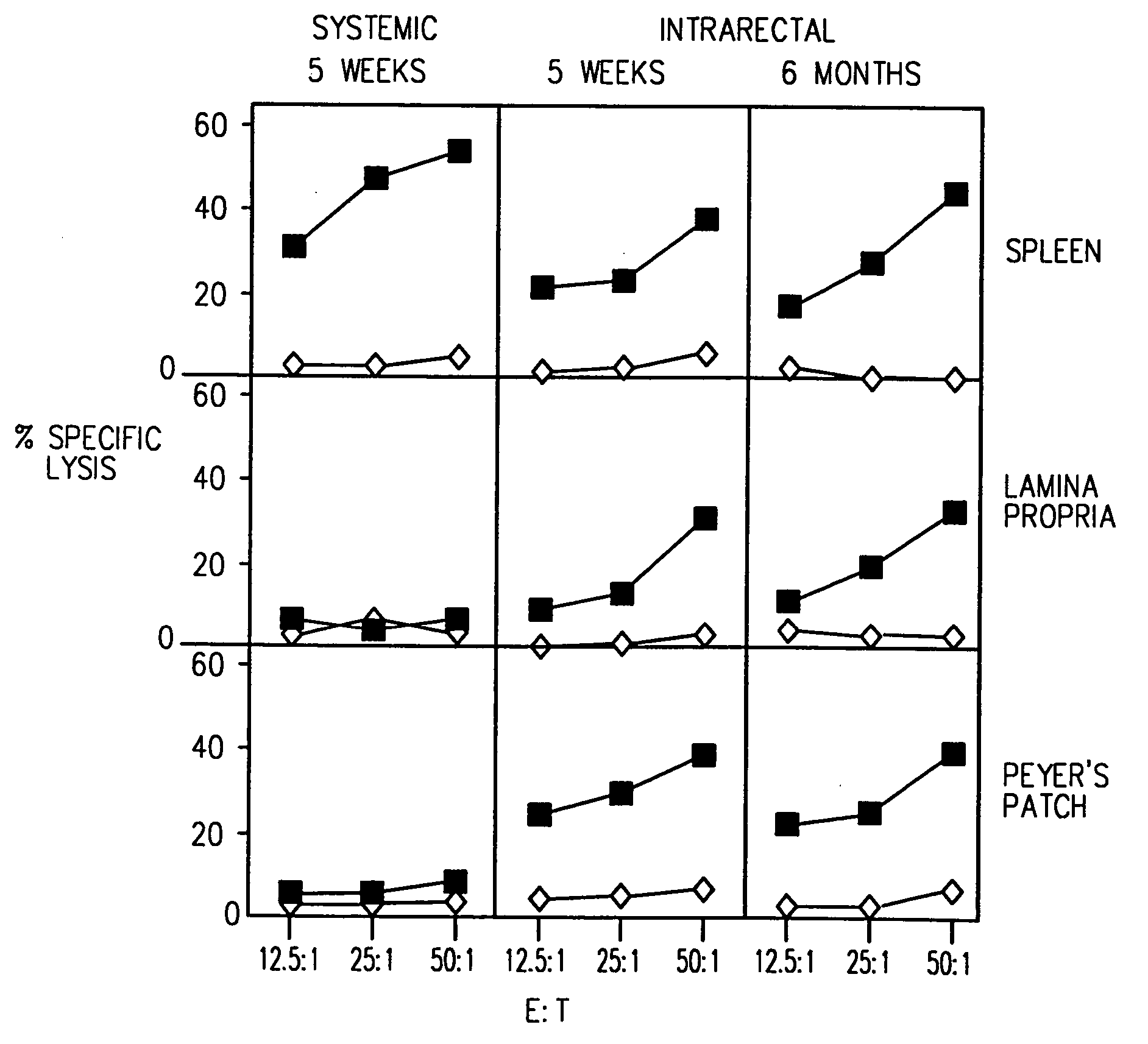
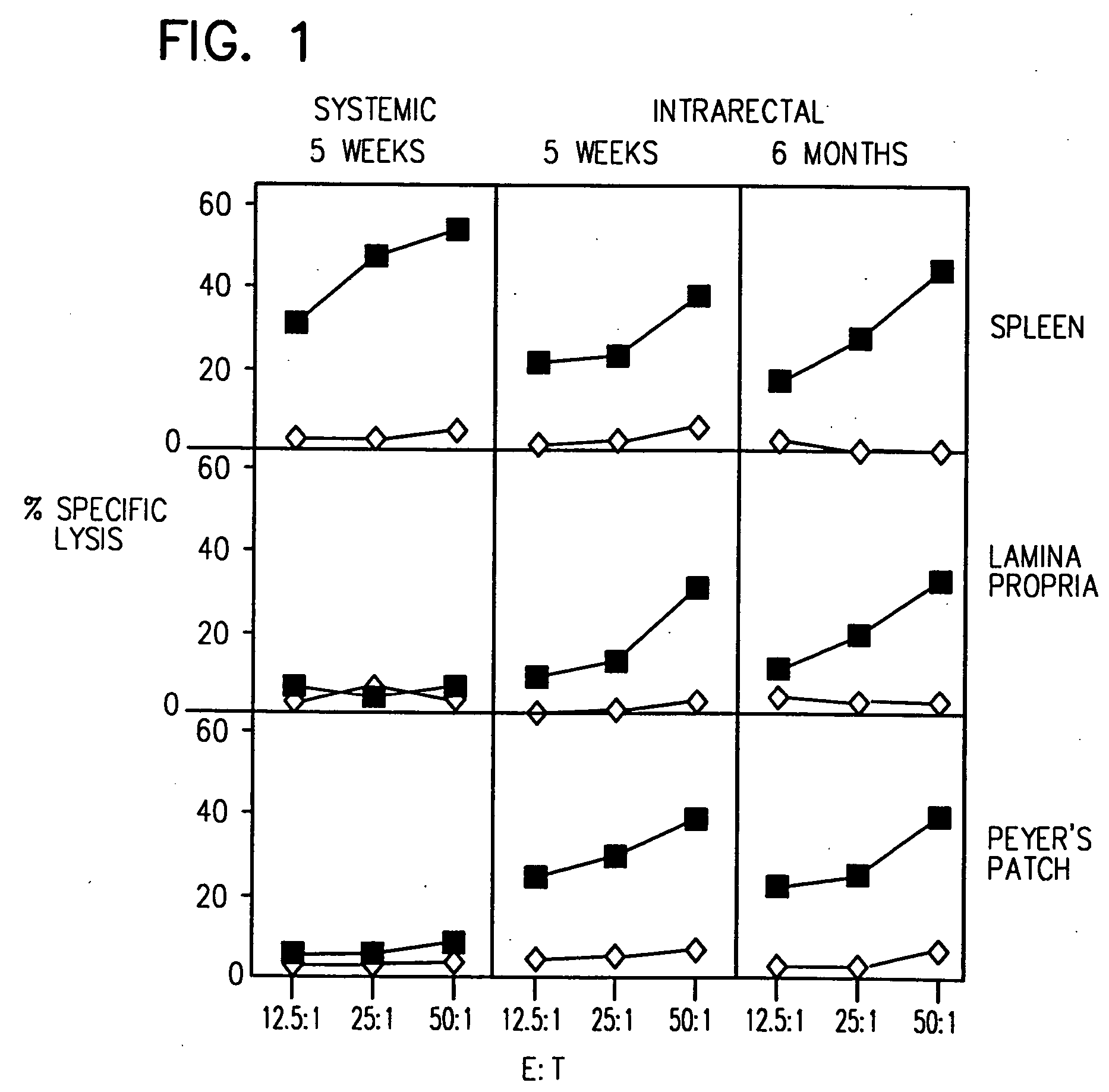
[0104] Mice were immunized IR with 4 doses (on days 0, 7, 14 and 21) of the HIV-1 CLUVAC PCLUS3-18IIIB (SEQ ID NO:2) (50 μg / mouse). 5 weeks to 6 months after the first dose, antigen-specific T cells were isolated from PP, LP and SP and tested for the presence of HIV-1 P18IIIB peptide specific CTL (FIG. 1), as described above. Closed squares show killing of P18IIIB-I10 (SEQ ID NO:16) -pulsed targets and open diamonds show killing on unpulsed targets. IR immunization induced long-lasting protective immune responses: antigen-specific CTL were detected in mucosal inductive (PP) and effector (LP) sites and in a systemic site (SP) at least 6 months after immunization. In contrast, systemic immunization (s.c. in incomplete Freund's adjuvant) induced CTL in the spleen but not in the mucosal immune system (i.e., PP and LP).
example 2
Comparison of CTL Responses with and without a Mucosal Adjuvant
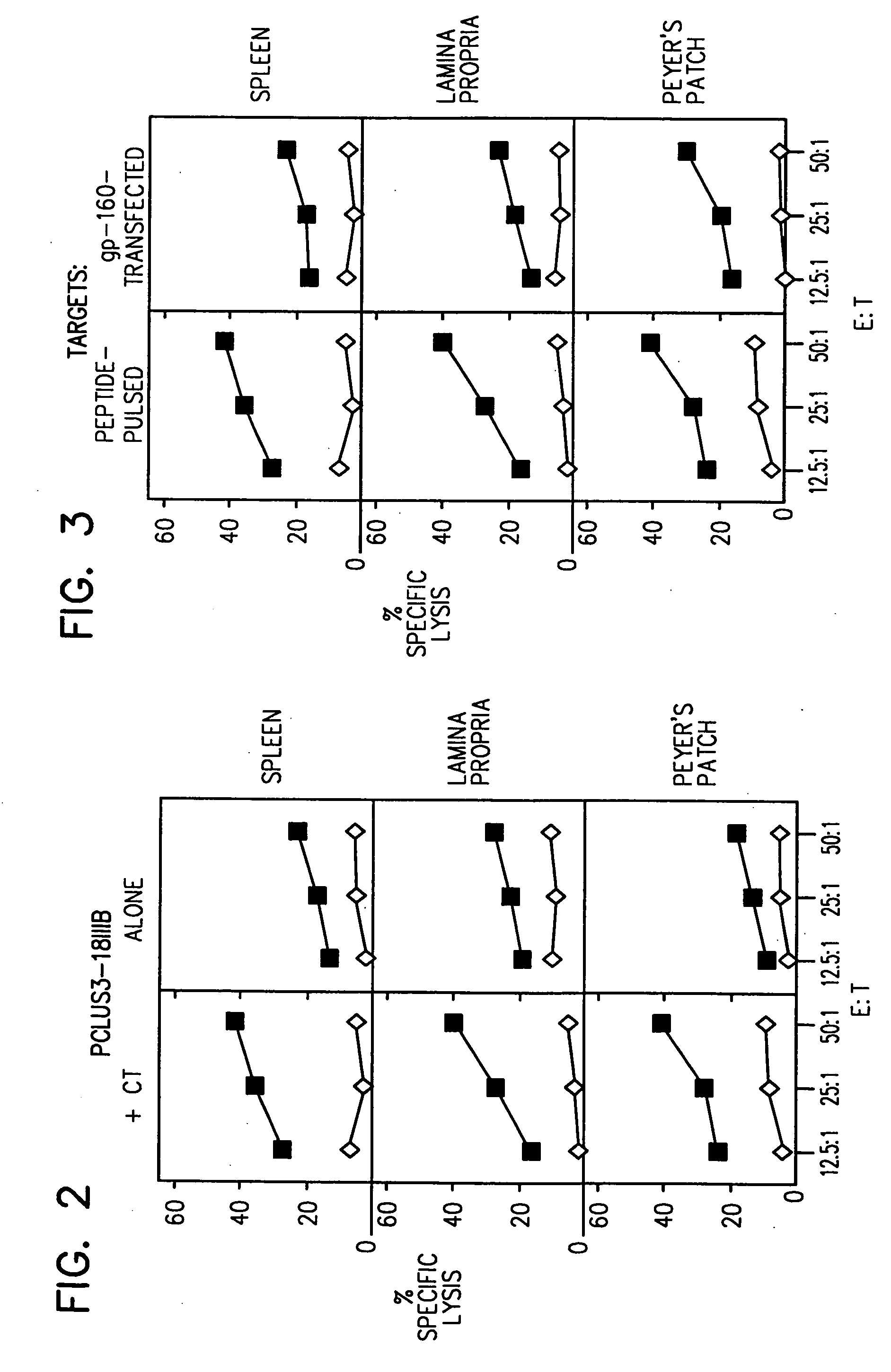
[0105] BALB / c mice were immunized IR with 4 doses of the synthetic HIV-1 CLUVAC PCLUS3-18IIIB (SEQ ID NO:2) (50 μg / mouse per immunization) alone, i.e., in the absence of adjuvant or cytokine on days 0, 7, 14 and 21. In parallel, another group of mice was immunized IR with PCLUS3-P18IIIB (SEQ ID NO:2) HIV-1 peptide in combination with CT (1 μg / mouse). On day 35 antigen-specific T cells were isolated from PP, LP and SP. Immune cells from SP, PP, or LP were cultured and tested for antigen specific CTL (FIG. 2), as described above. Closed squares show killing of P18IIIB-I10 (SEQ ID NO:16) pulsed targets, and open diamonds show killing of unpulsed targets. IR administration of peptide alone induced a significant response. The response was enhanced by the co-administration of CT.
example 3
CTL Induced by Mucosal Immunization Lyse Targets Expressing HIV-1 gp160 Envelope Protein
[0106] Mice were immunized IR with 4 doses (on days 0, 7, 14 and 21) of the HIV-1 CLUVAC PCLUS3-18IIIB (SEQ ID NO:2) (5 μg / mouse per immunization) in the presence of CT (1 μg / mouse). on day 35, antigen-specific T cells were isolated from PP, LP and SP. Immune cells from SP, PP, or LP were cultured as described above. Cytolytic activity of CTL was measured using a standard 51Cr release assay (FIG. 3).
[0107] Three different cell lines were used as target cells: 15-12 cells, 3T3 18 Neo BALB / c cells and P815 cells. 15-12 cells are BALB / c 3T3 fibroblasts transfected with a vector encoding HIV-1 gp160 (Takahashi et al. Proc. Natl. Acad. Sci. USA 85:3105 (1988)); 3T3 18 Neo BALB / c cells are BALB / c 3T3 fibroblasts transfected with an expression vector containing a Neor gene but no gp160 gene; and P815 cells are untransfected cells that present antigenic peptides added to the culture. CTL lysis of gp160...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Solubility (mass) | aaaaa | aaaaa |
| Immunogenicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com