Methods of implating mesenchymal stem cells for tissue repair and formation
a mesenchymal stem cell and tissue repair technology, applied in the field of mesenchymal stem cell isolating and transplanting mesenchymal stem cells for tissue repair or formation, can solve the problems of unable to achieve the desired goal, cells may lose their multipotency, and both methods are very difficult to be reproduced by other labs, so as to stimulate or enhance tissue repair, maintain or increase bone volume, or bone strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Isolation of hMSCs and Flow Cytometry:
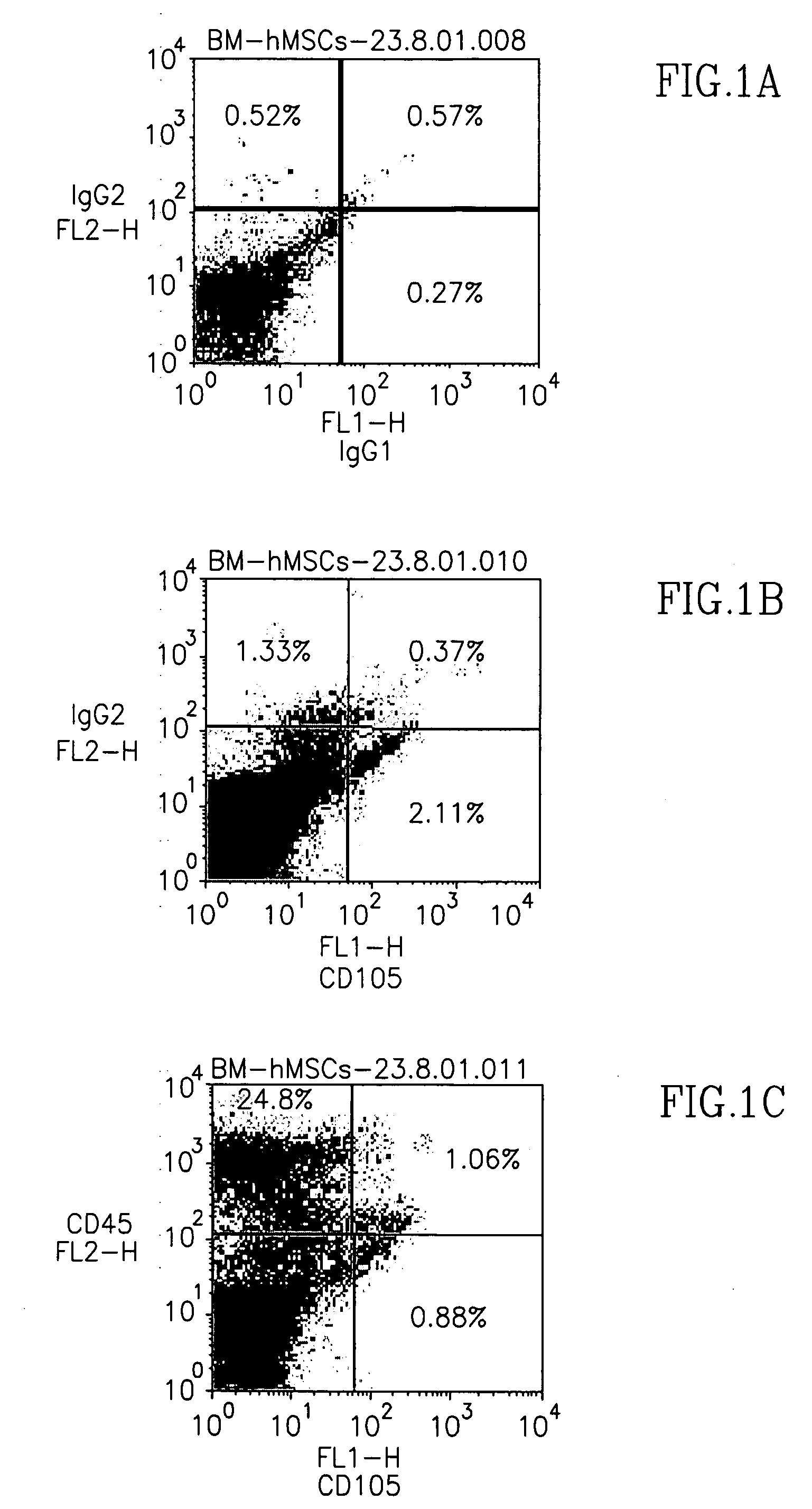
[0076] In order to characterize the CD105+ cell population in human bone marrow (BM), human BM-derived mononuclear cells were analyzed for co-expression of CD105 antigen and other hematopoietic markers. Marker FACS analysis of freshly separated human BM mononuclear cells demonstrated that CD105 was co-expressed with other hematopoietic markers at low levels, except for CD45, which was co-expressed at higher percentages on CD105 cells (about 40%, FIG. 1C). More importantly, however, was the minimal expression of CD14 (a monocyte marker) on CD105+ cells, indicating that macrophages, the hematopoietic cell population from bone marrow with the greatest adherence to plastic, did not comprise the CD105+ cell population isolated (FIG. 1E).
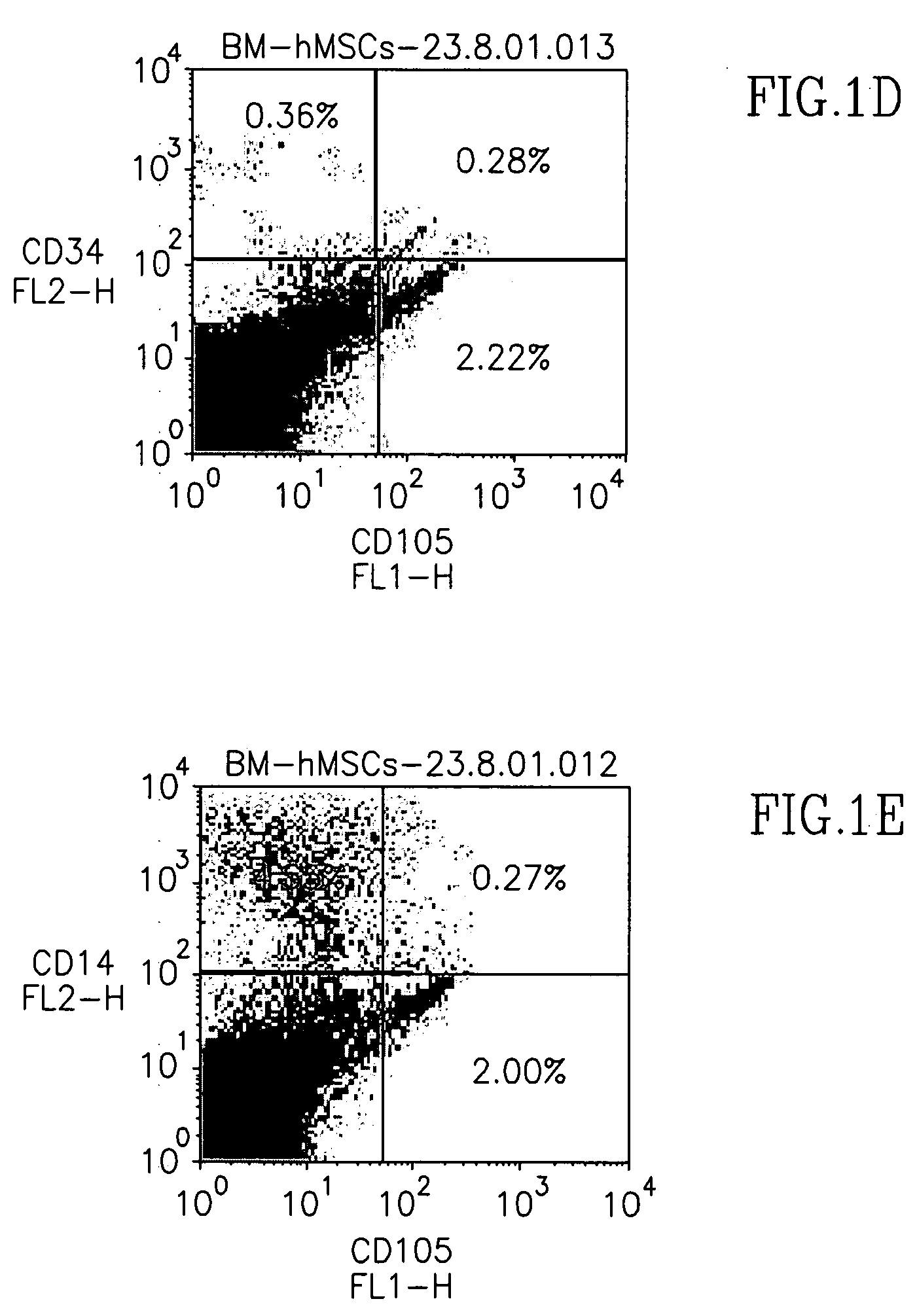
[0077] CD105+ cells were isolated from human BM mononuclear cells via CD105-microbeads, then plated for expansion. Colonies of fibroblastic-like cells appeared within 7-10 days following initial plating, and were a...
example 2
In vitro osteogenic differentiation:
[0078] In order to determine the in vitro osteogenic potential of CD105+ cells, CD105+ cells were cultured following expansion (passages 3-5) under conditions inducing differentiation of mesenchymal stem cells to cells of osteogenic lineage (Pittenger et al., 1999). Significant increase in alkaline phosphatase activity was evident in induced hMSCs (with supplement), as compared to non-induced hMSCs (without supplement), during the 3 weeks of differentiation (FIG. 3A). Continual, significant increase in calcium deposition (expressed as OD at 575 nm) was observed during the differentiation period (1, 2, and 3 weeks, FIG. 3B).
example 3
Non-Culture Expanded, CD105+ hMSCs Form Cartilage and Bone in vivo:
[0079] Freshly isolated, non-culture expanded hMSCs isolated via CD105 microbead separation were transplanted into skull-defects induced in CD-1 nude mice, in order to determine their in vivo osteogenic potential. New bone formation was observed in sections processed and stained with H&E, whereas no evidence of newly formed bone was observed in similarly injured mice transplanted with collagen inserts containing CD105− cells (FIG. 4) or collagen inserts alone (data not shown).
[0080] Osteogenic differentiation of non-culture expanded hMSCs in vivo was also demonstrated via implanting the cells into NOD / SCID mice. Non-culture expanded hMSCs (1-1.5×106 cells) labeled with DiI were mixed with rhBMP2, loaded onto collagen sponges (Duragen) serving as scaffolding and transplanted under the skin of NOD / SCID mice. Cartilage and bone formation was detected in harvested implants, two weeks post-transplantation (FIGS. 5A and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| periods of time | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com