Method for obtaining a TGF-beta enriched protein fraction in activated form, protein fraction and therapeutic applications
a technology of tgf-beta and protein fraction, applied in the field of tgf-beta factor purification, can solve the problems of reducing the final yield, long and tedious tgf-beta purification process, and not having a process to purify tgf-beta
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Preparation of a Solution Rich in Whey Proteins (WPI) as a Starting Product for the Process According to the Invention
[0166] 10,000 Kg of skimmed milk with a content in dry matter of 92.9 g / Kg and a content in N×6.38 of 35.4 g / Kg were introduced, at 50° C., in a microfiltration equipment comprising a 4.6 m2 and 0.1 μm Membralo® membrane (alumine-zircone) and functioning with co-current recirculation of the microfiltrate such as to obtain a uniform transmembrane pressure of 0.6 to 0.7 bars.
[0167] The scanning rate in the Retentate compartment was fixed at 7 m / s.
[0168] The microfiltrate extraction flux was fixed at 345 l / h. The retentate extraction flux was fixed at 172.5 l / h.
[0169] The 6670 l of microfiltrate obtained, with a content in dry matter of 57.8 g / Kg and a content in N×6.38 of 6.4 g / Kg, were cooled down at 10° C. and introduced in an ultrafiltration equipment comprising a 9.6 m2 Koch® membrane, with a spiral conception, and having a cut-off of 5 Kd. The outlet pressure...
example ii
Preparation of a Fraction Highly Enriched in TGF-Beta Starting from a Solution Rich in Whey Proteins
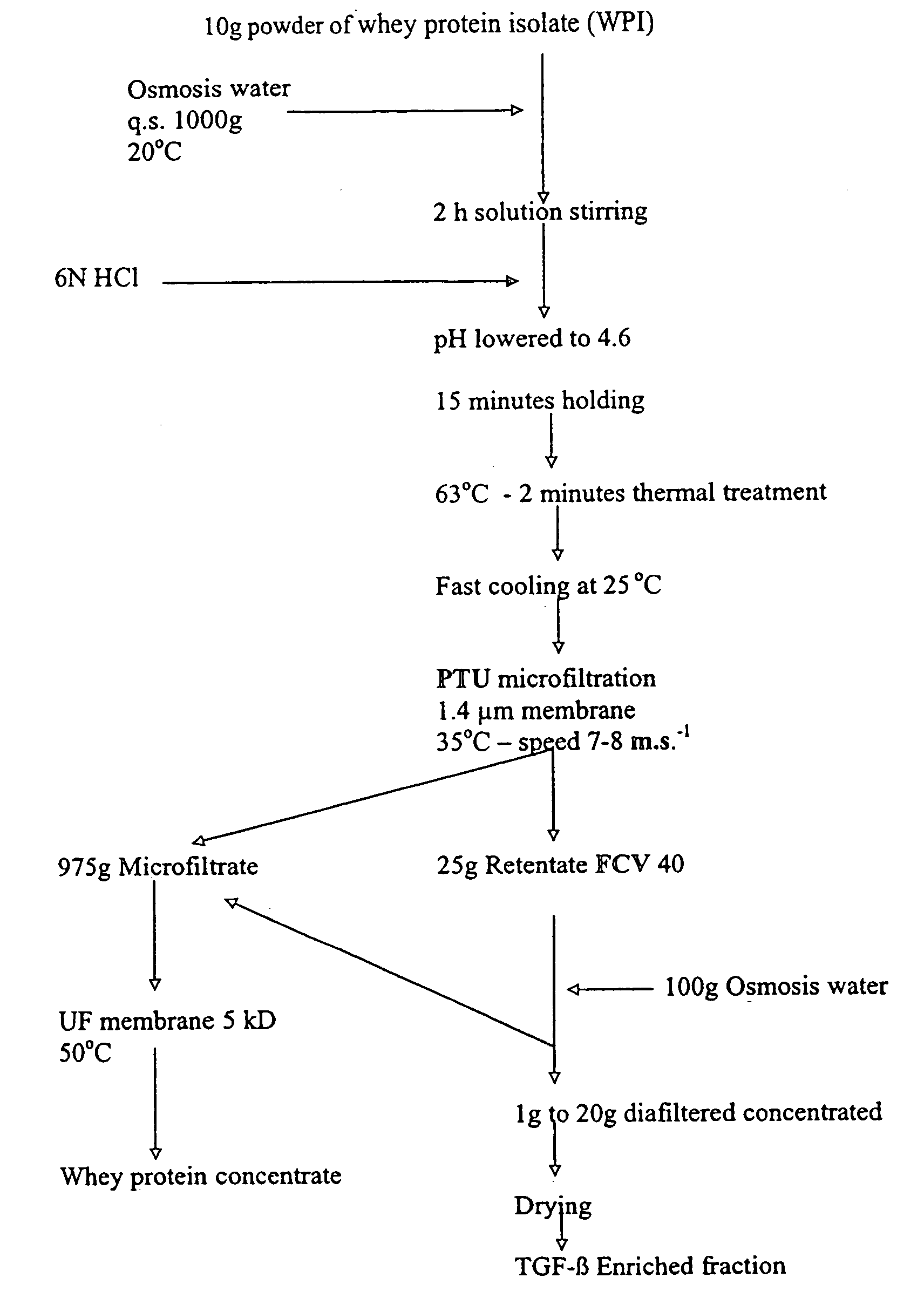
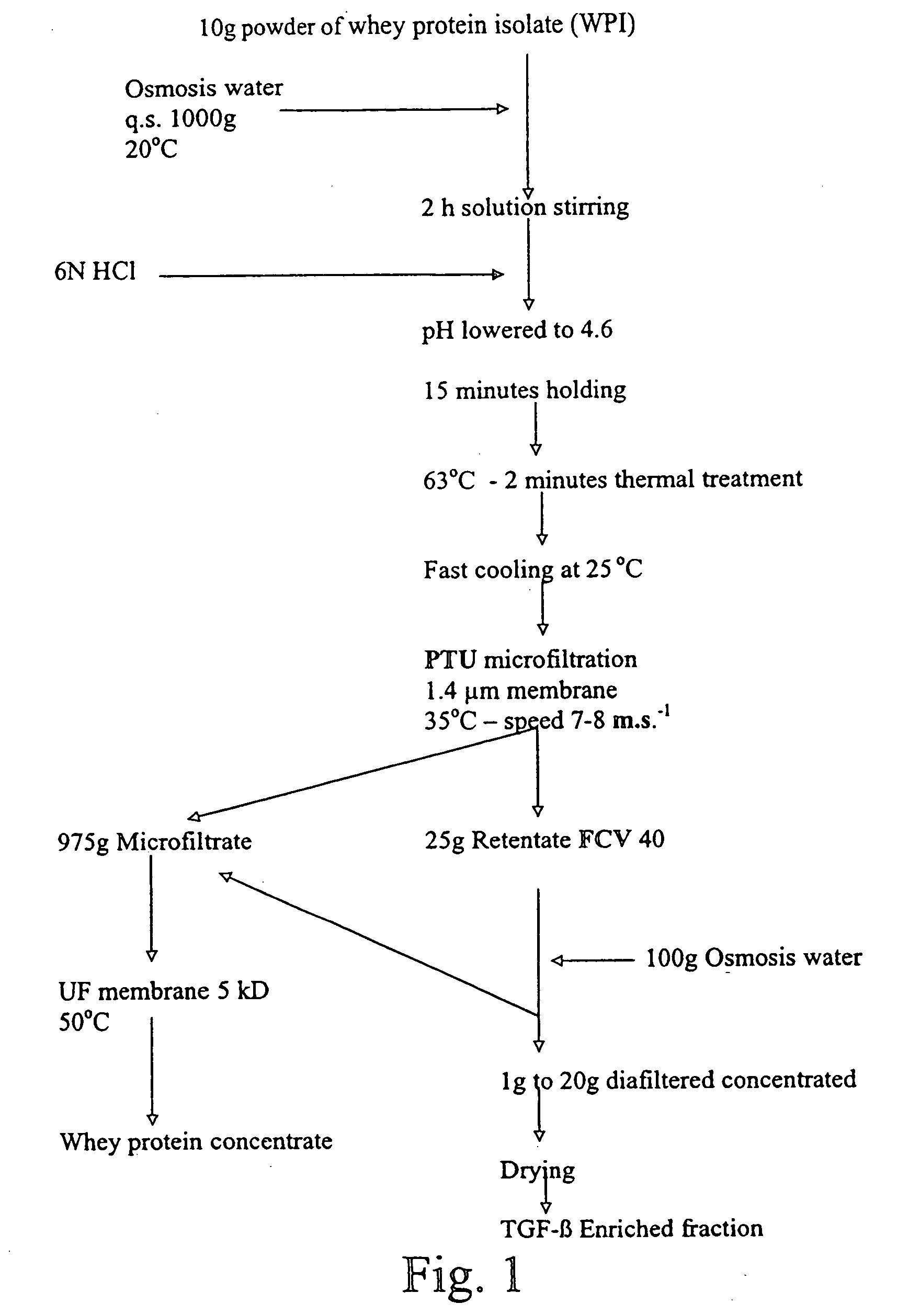
[0172] 200 Kg of the diafiltered retentate obtained according to Example I were diluted in 2000 l with osmosis water at a temperature of 20° C. in a tank equipped with a balde agitator.
[0173] After 10 minutes of stirring, 1800 ml of 6N HCl were progressively added until the lowering of the pH value from 7.25 to 4.6. Stirring was continued during 10-15 minutes. The solution was thermally treated at 63° C.-2 minutes and cooled down at 25° C. in an Actijoulee equipment by 1000 l aliquots. The 2000 l of the obtained suspension, heated at 35° C., were then microfiltered in continuous in an equipment with 4.6 m2 STERILOX® membranes having a 1.4 μm average pore diameter with co-current recirculation of the microfiltrate in a manner to obtain a uniform transmembrane pressure between 0.4 and 0.8 bars in 4.5 hours.
[0174] The extraction flux of the microfiltrate was fixed at 400 l / h. No extr...
example iii
Quantitative and Qualitative Analysis of the Protein Fraction Highly Enriched in TGF-Beta According to the Invention
[0177] 1 mg of the lyophilized powder obtained in the Example II was dissolved in 1 ml of milliQ water then diluted 5 times in buffer A. The analytical equipment used was a Waters 600 E HPLC chromatograph with a “source RPC 3 ml” column (Pharmacia®).
[0178] The two buffers used were: [0179] Buffer A: Trifluoroacetic acid (TFA) 0.1%, and [0180] Buffer B: TFA 0.09% in acetonitrile 90%.
[0181] 50 μl of product were injected and the elution was carried out by a gradient of 30 to 100% of buffer B in 30 minutes with a flux of 2 ml / min (at room temperature). Detection was carried out at 214 nm. Treatment of chromatographic areas was carried out with a Nelson® software, which allowed the estimation of the content, when compared to the total proteins: 53% of alpha-lactalbumin, 0.03% of serum-albumin, 10.9% of beta-lactoglobulin and 18.3% of immunoglobulins.
[0182] The TGF-bet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com