Metal alloy and metal alloy storage product for storing fast neutron emitters
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
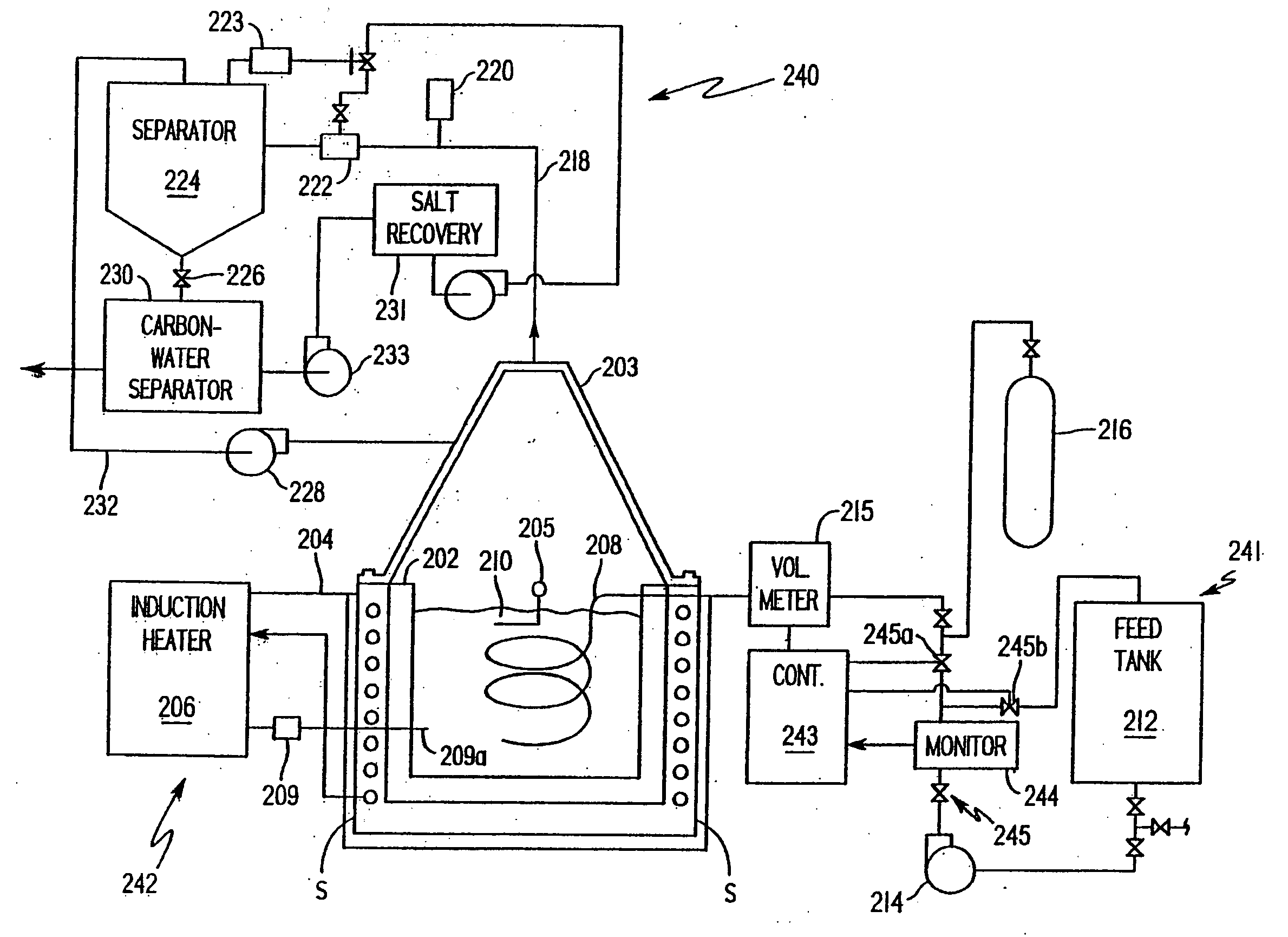
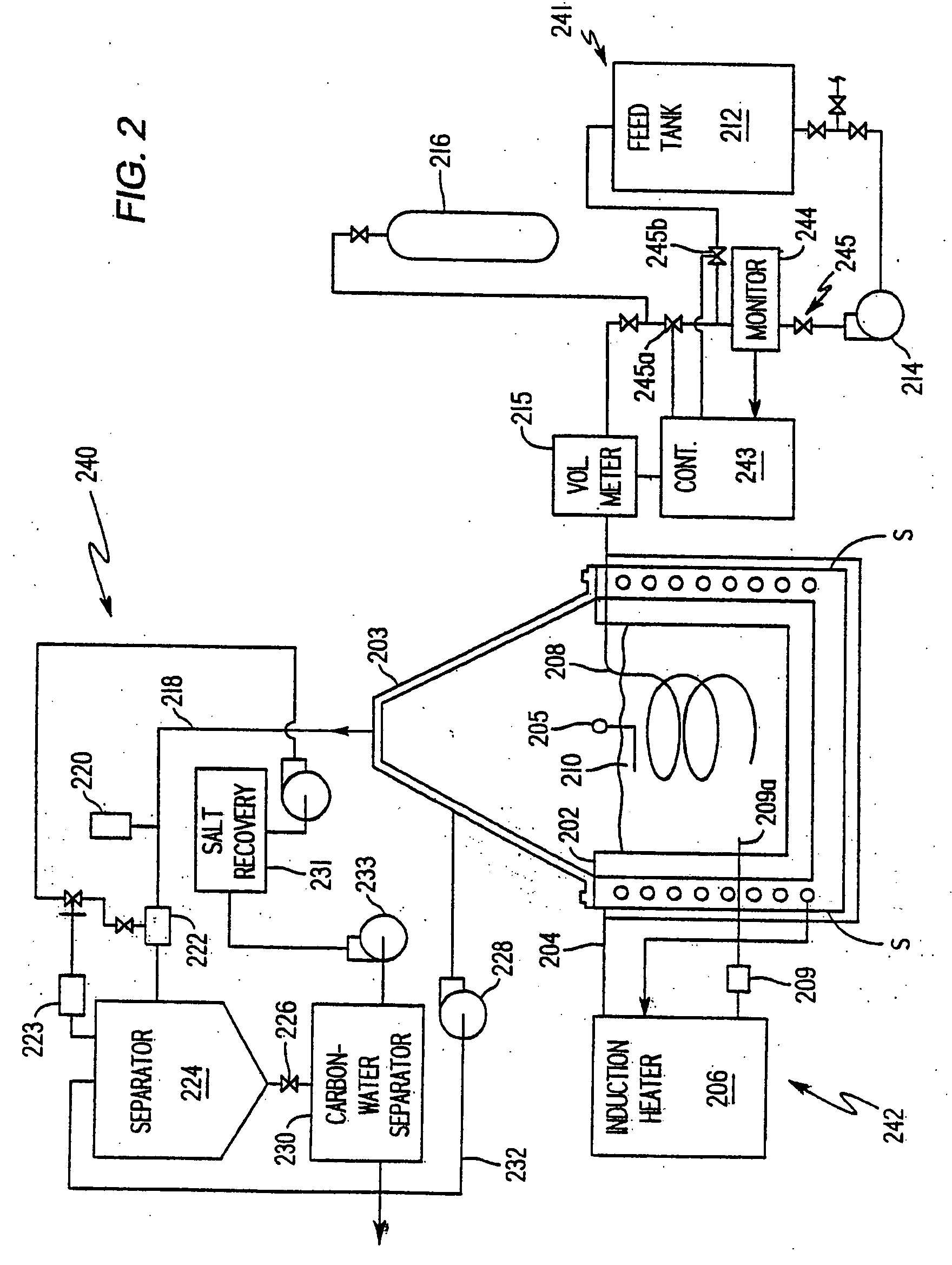
[0065] A waste material is analyzed with a mass spectrometer and found to comprise thorium 229 at 9 parts per million (ppm), PCBs at 500 ppm, and creosote at 1000 ppm in water. To treat one ton of the waste material, a liquid metal alloy according to the invention may include predominantly aluminum and perhaps small percentages of zinc, iron, copper, and calcium. The primary emissions of thorium 229 include alpha particles at 5.168 MeV. Beryllium 11 is added to the chemically active fraction as a corresponding absorber for the alpha emissions and lead 206 is added to absorb the primary gamma emissions from the thorium 229 and secondary gamma emissions as the alpha particles interact with materials in the bath. The 9 ppm of thorium 229 equates to 6.412 grams of the isotope per ton of the waste material. 6.42 kilograms of beryllium 11 is included in the metal bath to provide a one thousand to one correspondence between the beryllium and the expected alpha emissions. 12.84 kilograms of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Radioactivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com