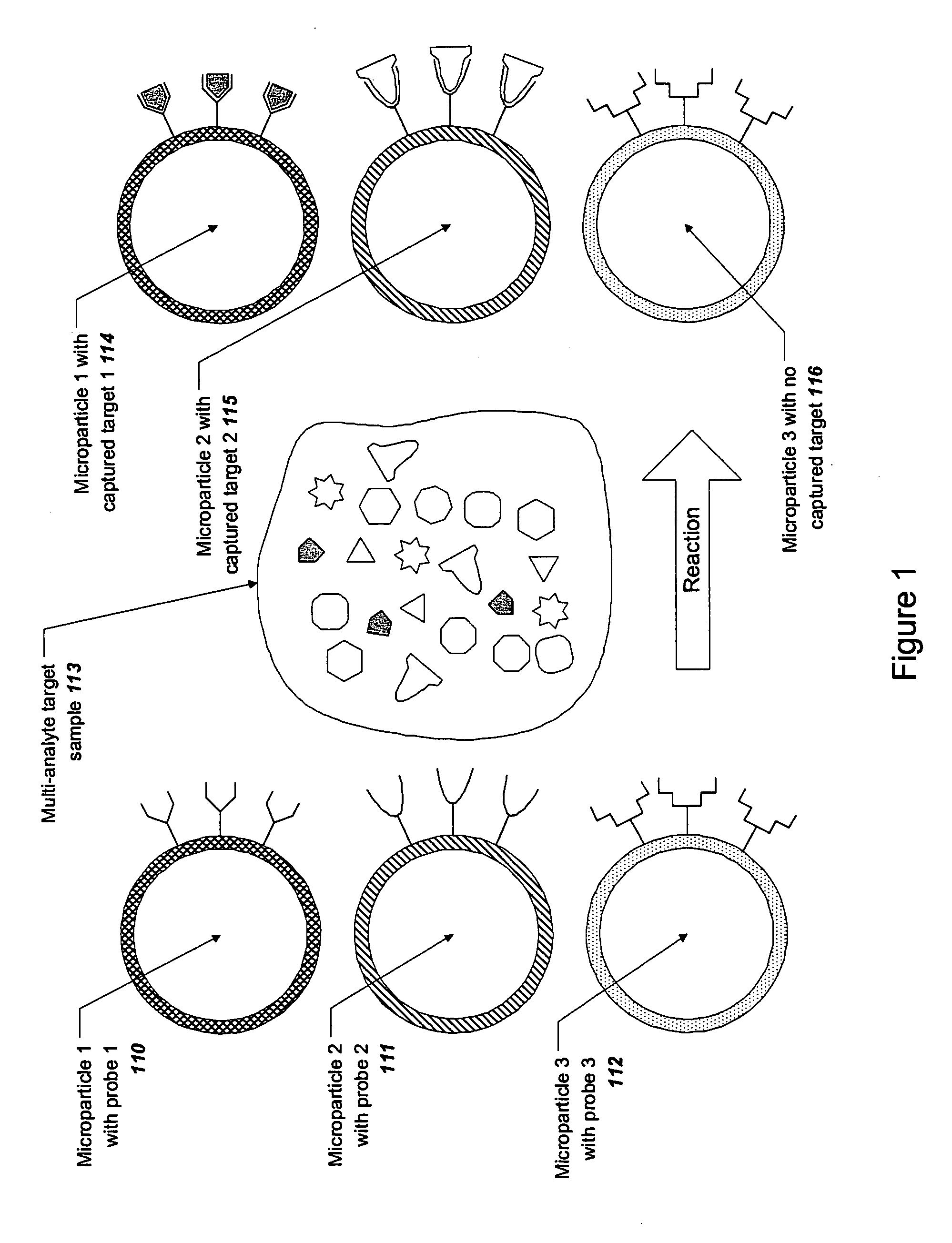
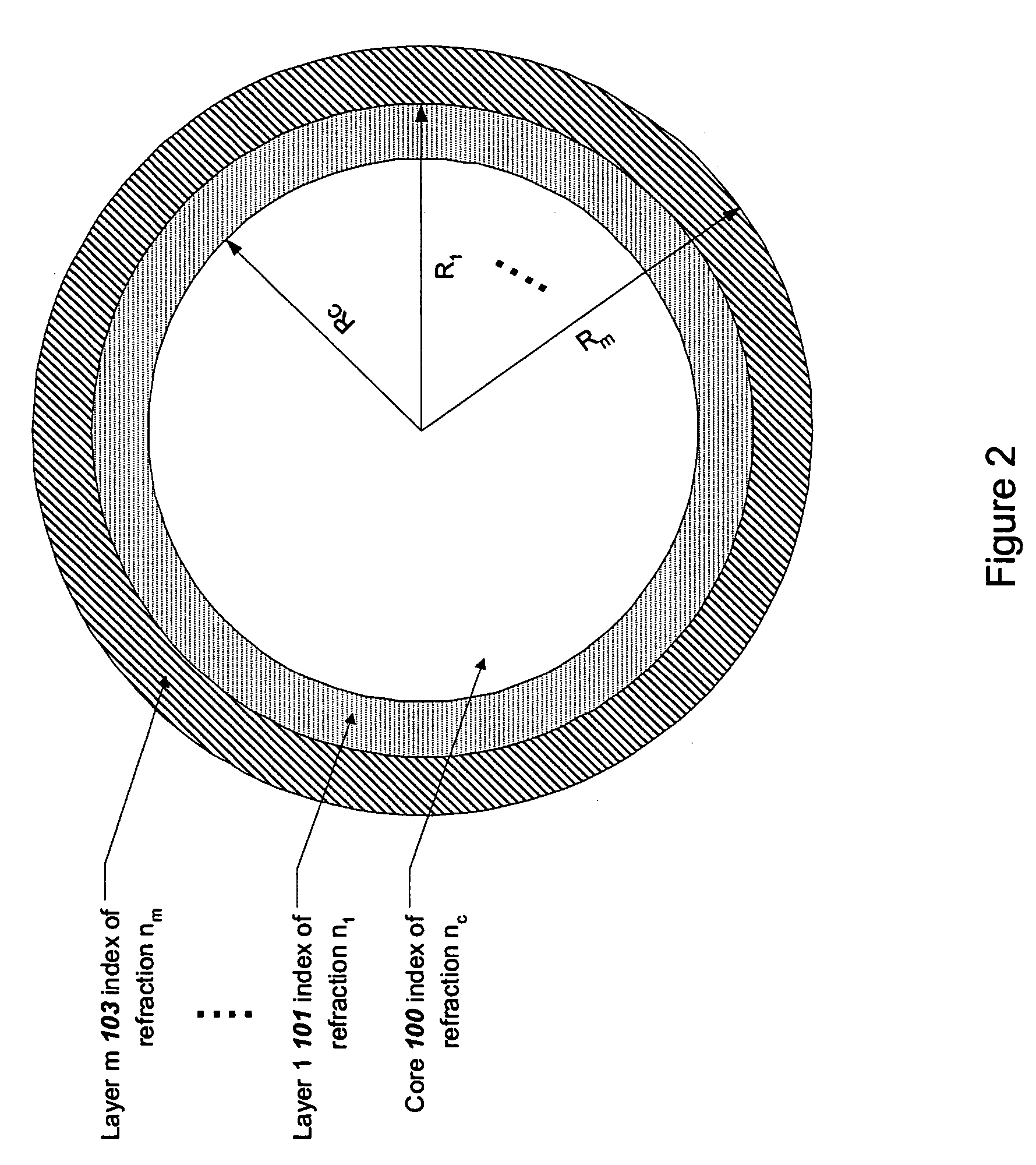
Microparticle-based methods and systems and applications thereof
a microparticle and microparticle technology, applied in the field of microparticle-based measurement methods and systems, can solve the problems of slow diffusion of analytes to and on the surface, inability to efficiently control the binding kinetics of microarrays, and inability to cover relatively large diffusion distances of analytes,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Identification of Individual Glass Microparticles by Resonant Light Scattering
[0290] The purpose of this Example was to demonstrate the identification of individual glass microparticles using resonant light scattering spectra using an optical probe system.
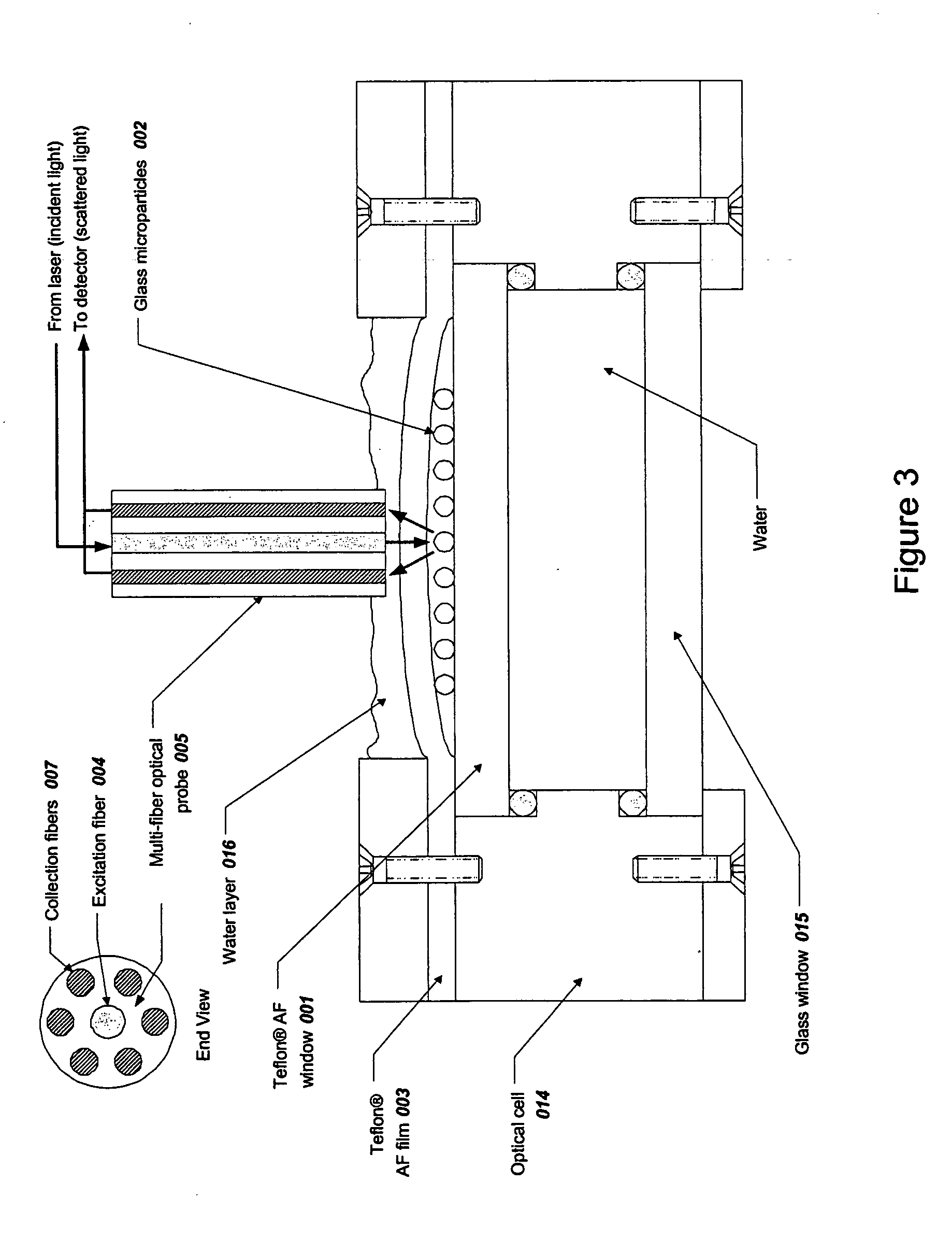
[0291] Spherical glass microparticles of refractive index approximately 1.9 and diameter approximately 35-40 μm (MO-Sci Corp., Rolla, Mo., Product number GL-0175, Lot 7289552-S1) were used without further treatment. Approximately 0.5 g of particles was placed in approximately 10 mL of distilled water and a suspension was created using a magnetic stir bar. A sample of approximately 0.1 mL of this suspension was placed on the top Teflon® AF 2400 (DuPont Co., Wilmington, Del.) window of thickness about 2 mm 001 as shown in FIG. 3. The suspension was optionally covered with a Teflon® AF 2400 cover film (about 0.05 to 0.13 mm thickness) 003 and the glass microparticles 002 were allowed to settle to the upper surface of the top window ...
example 2
Identification of a Multiplicity of Glass Microparticles by Resonant Light Scattering Imaging
[0293] The purpose of this Example was to demonstrate the identification of a multiplicity of glass microparticles by resonant light scattering using a spectral imaging system.
[0294] The spectral imaging system shown in FIG. 7 was used to acquire and process resonant light scattering spectra from multiple glass microspheres simultaneously. The optical cell of FIG. 3 was modified as shown in FIG. 6 by replacing the water underneath the top Teflon® AF 2400 window with an ink absorber solution 018, which consisted of 1 part ink (Higgins Fountain Pen India Ink, Item 46030-723, Sanford Co., Bellwood, Ill.) to 100 parts distilled water. The ink solution served to absorb any incident light that had not interacted with the microspheres, thereby reducing optical noise from scattering and back reflection. The imaging optical cell 019 was loaded with glass microparticles 002 as in Example 1 and place...
example 3
Detection of Binding of Avidin on Biotinylated Microparticles Using Resonant Light Scattering
[0309] The purpose of this Example was to demonstrate detection of avidin binding to biotinylated glass microparticles using resonant light scattering. Amine groups were introduced on the surface of glass microparticles using silane chemistry. Then, the microparticles were biotinylated by reaction with Sulfo-NHS-SS Biotin. Avidin binding to the biotinylated microparticles was detected using resonant light scattering. Reversibility of the binding signal upon cleaving the avidin from the particle was also demonstrated.
[0310] A. Surface Preparation of Microparticles:
[0311] High-refractive index glass microparticles (Mo-Sci Corp., Rolla, Mo., Product number GL-0175, Lot 7289552-S1, refractive index approximately 1.9) were first cleaned by leaching with 0.5 M HNO3 at room temperature for 5 min. The glass particles were then treated with the following series of steps: a deionized water rinse, t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical wavelengths | aaaaa | aaaaa |
| optical wavelengths | aaaaa | aaaaa |
| optical wavelengths | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com