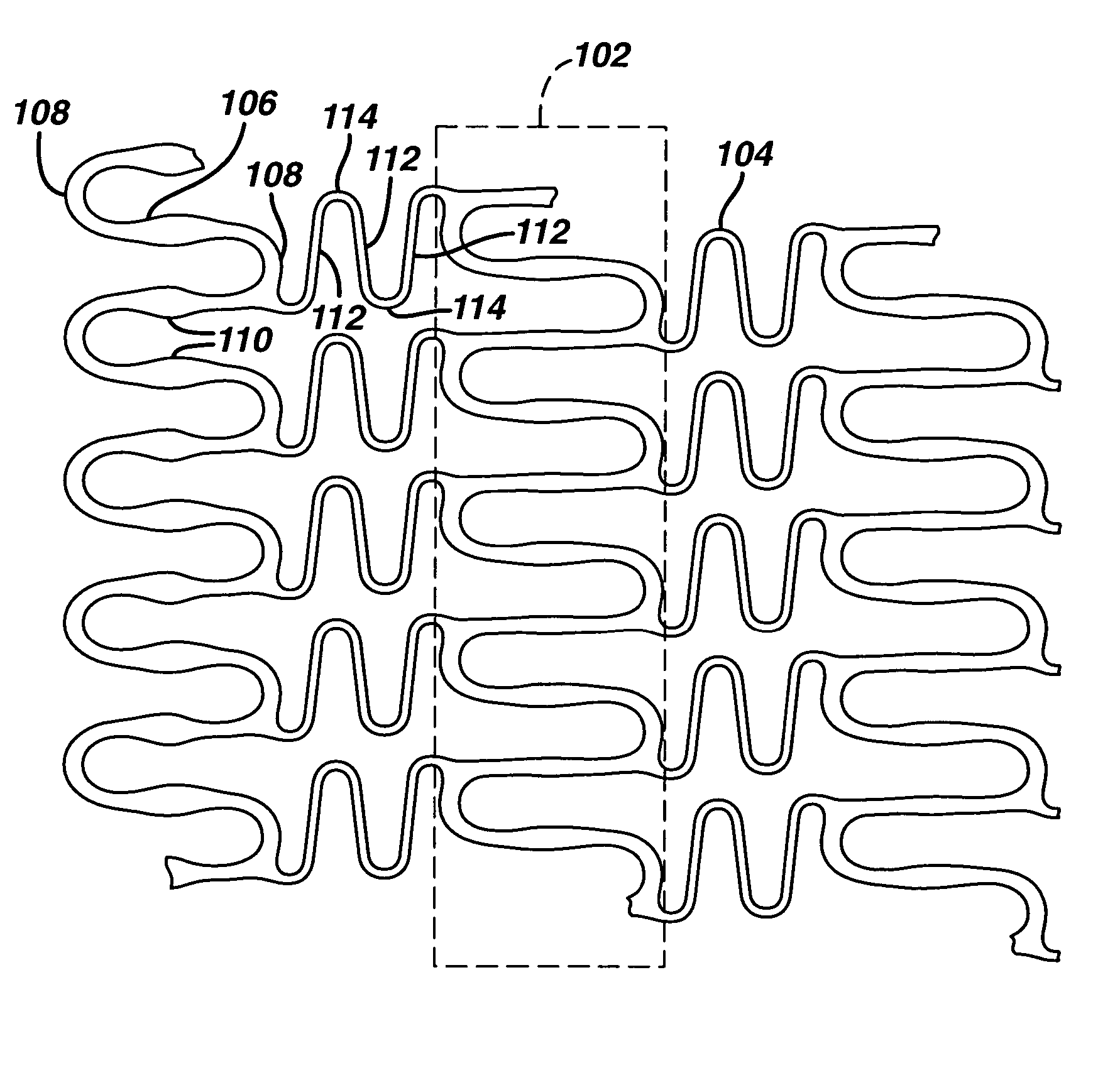
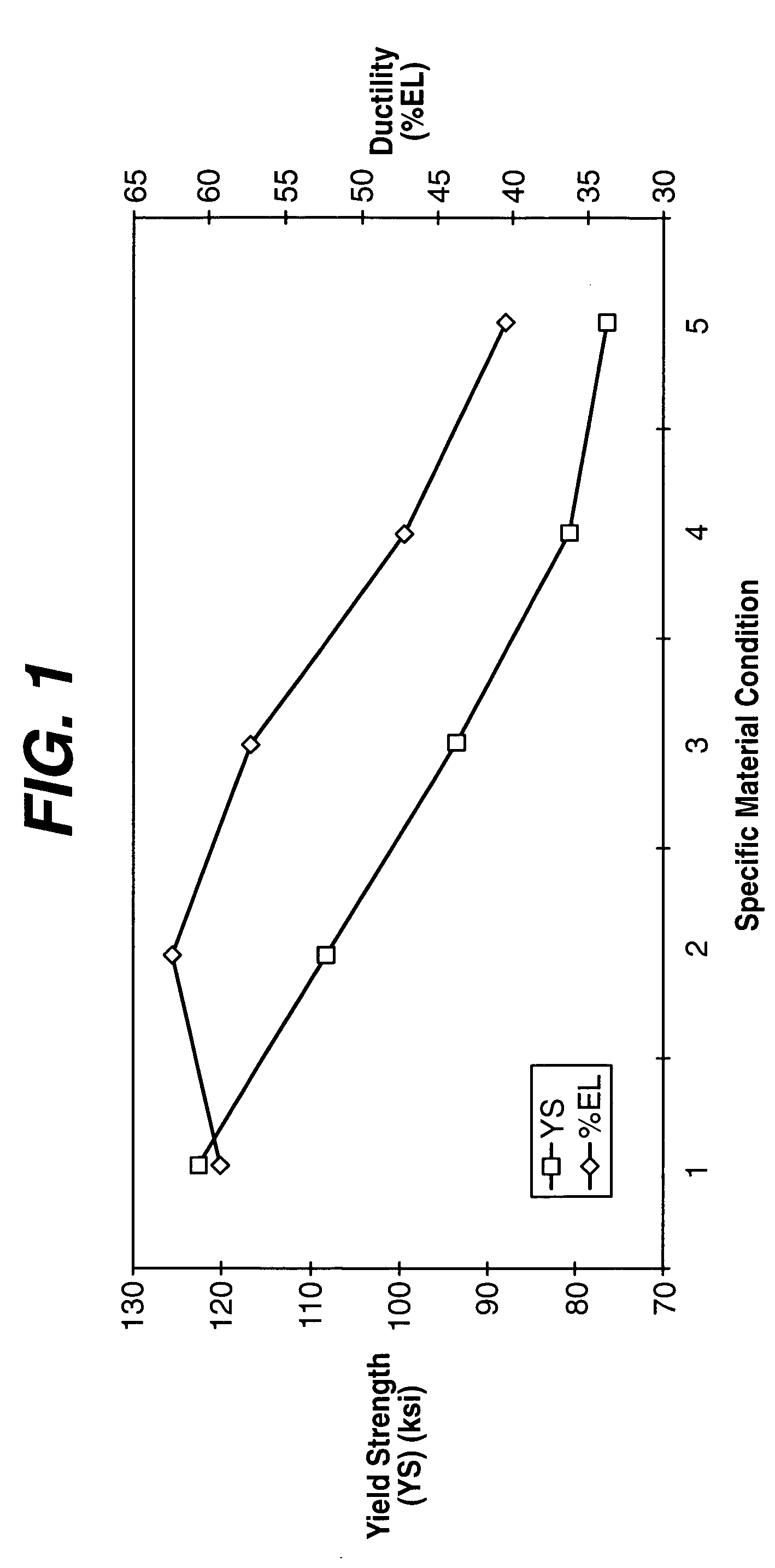
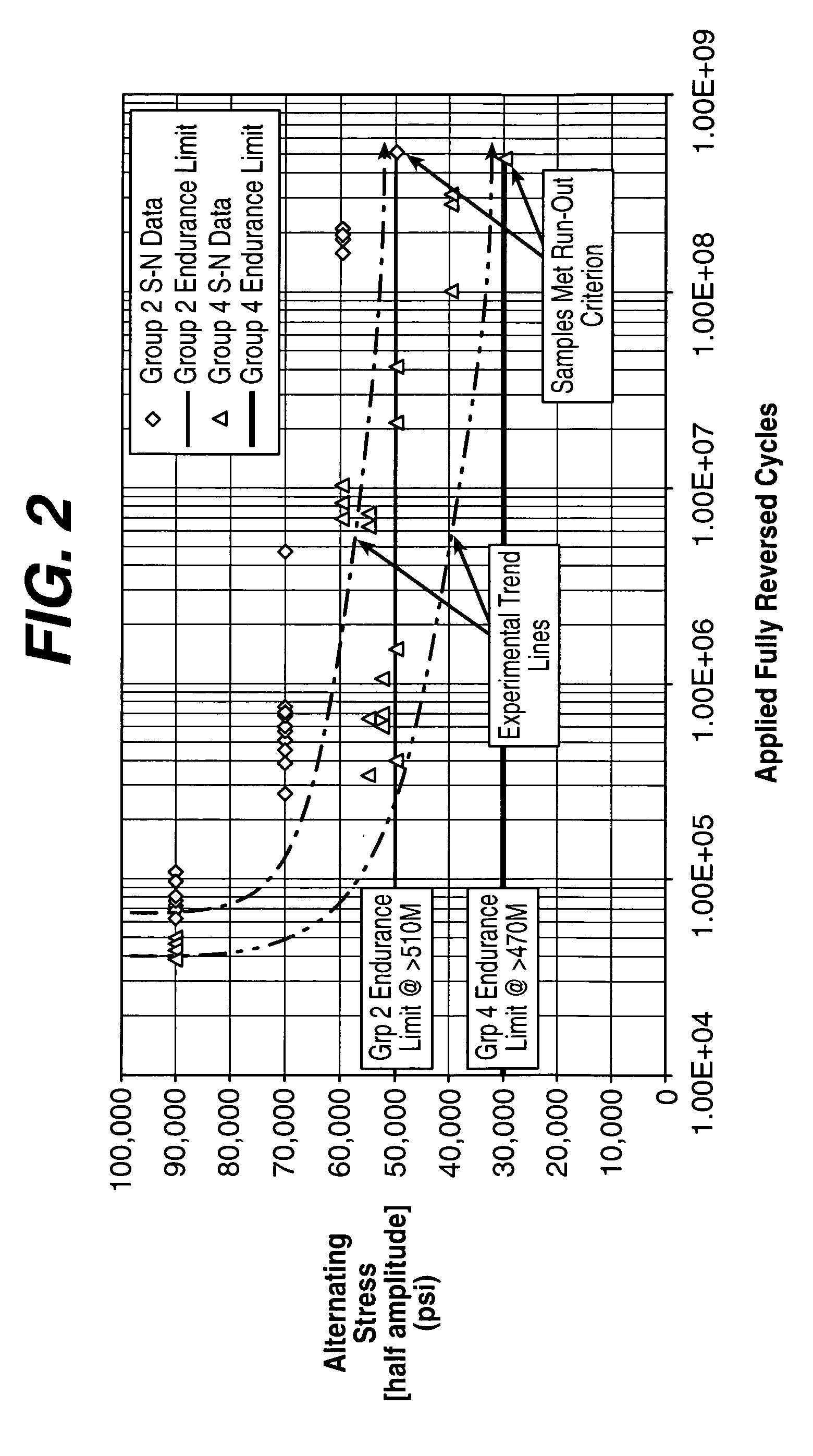
Material for high strength, controlled recoil stent
a recoil stent and high strength technology, applied in the field of alloys, can solve the problems of inadequate microstructural tailoring of intravascular stents, and achieve the effect of simple and inexpensive manufacturing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0016] Biocompatible, solid-solution strengthened alloys such as iron-based alloys, cobalt-based alloys and titanium-based alloys as well as refractory metals and refractory-based alloys may be utilized in the manufacture of any number of implantable medical devices. The biocompatible alloy for implantable medical devices in accordance with the present invention offers a number of advantages over currently utilized medical grade alloys. The advantages include the ability to engineer the underlying microstructure in order to sufficiently perform as intended by the designer without the limitations of currently utilized materials and manufacturing methodologies.
[0017] For reference, a traditional stainless steel alloy such as 316L (i.e. UNS S31603) which is broadly utilized as an implantable, biocompatible device material may comprise chromium (Cr) in the range from about 16 to 18 wt. %, nickel (Ni) in the range from about 10 to 14 wt. %, molybdenum (Mo) in the range from about 2 to 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| wall thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com