Amplification method for solid phase immunoassays
a solid-phase immunoassay and amplification method technology, applied in the field of immunoassays, can solve the problems of increasing the labor and cost of manufacturing the test device, limiting the processing, and reducing so as to achieve the effect of improving the sensitivity of the devi
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Labeled Antibodies
[0024] Antibodies specific for influenza A antigens and antibodies specific for influenza B antigens were conjugated with detectable labeling moieties. Either distinguishable labels or the same label can be used to label each different antibody. Suitable labels include, but are not limited to, metal sols, latex particles, enzymes, fluorescent molecules, and the like, as known in the art. One label is carboxylate-modified latex particles (Seradyn, Indianapolis Ind.) available in various colors. Antibodies were coupled to carboxylate-modified latex particles using carbodiimide N-hydroxysuccinimide linkage, by standard procedures known in the art. Separate monoclonal antibody products from those used to prepare biotinylated reagents can be used, if available, or the same antibody product can be used for biotin-conjugation and for conjugation with a labeling moiety.
[0025] The labeled antibodies were vacuum dried by methods known in the art within a porous material, ...
example 3
Test Strip with Test Site and Optional Control Site
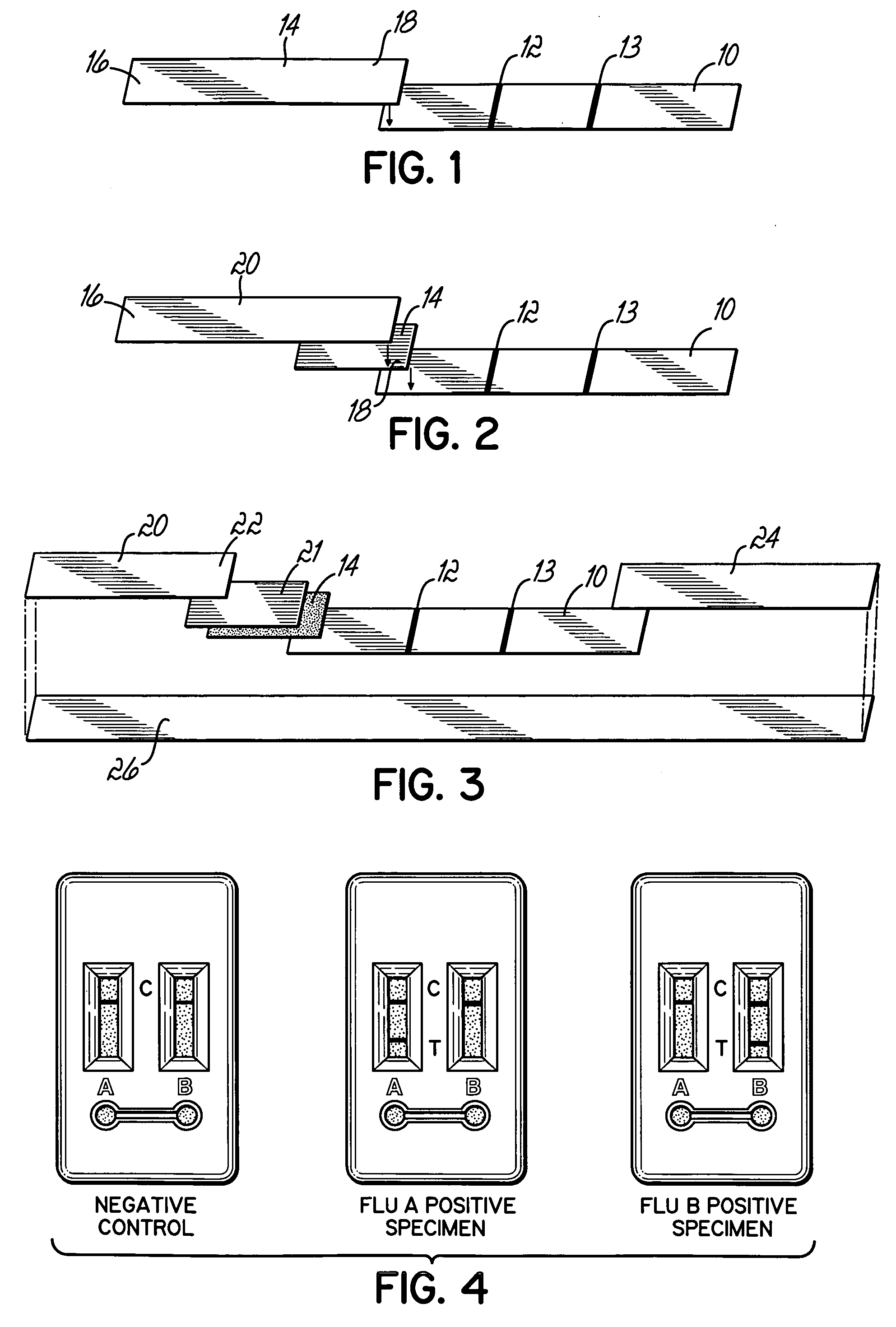
[0026] Dextran-polystreptavidin was immobilized as a capture reagent at a test site on a porous material. In one embodiment, the porous material was nitrocellulose, such as Millipore HiFlow Plus HF12002 or HF13502 (Millipore, Billerica Mass.). Dextran-polystreptavidin reagent was mixed in phosphate buffed saline (PBS) supplemented with sucrose (approximately 2.5%) and EDTA (approximately 0.33 mM), and deposited in one or more discrete bands or zones on the porous material, and allowed to dry or “cure” for about 10 to 15 hours,at 60° C. or about 7 to 14 days at room temperature. Although these time and temperature conditions were sufficient to immobilize the dextran-polystreptavidin conjugate on the porous material, they are exemplary and the invention is not limited to these specific conditions, but includes other conditions that result in immobilization
[0027] A simple method for checking the quality of immobilization was to add ...
example 4
Assembling the Composite Strip
[0029] As shown in FIG. 3, various components comprising the composite strip were anchored or secured on a backing support 26, such as an adhesive backing material, for example, white vinyl having a thickness of about 0.001 inches to about 0.03 inches. Such material is available from numerous sources. The first porous material 10 of the composite test strip, containing at least one test site 12, and optionally containing a control site 13, was positioned downstream of a conjugate pad 14 containing the labeled conjugate. The labeled conjugate pad 14 must overlap or make contact with the test strip 10 such that fluid possibly containing a target analyte moves laterally through the labeled conjugate pad 14 and through the test site 12. The use of an absorbent material 24 at the far downstream end of the first porous material 10 facilitated wicking or capillary flow through the test site 12 and acted as a sink to contain the fluid sample. Optionally, a bl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com