Oligonucleotides labeled with stable isotopes and a method for detecting the same
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
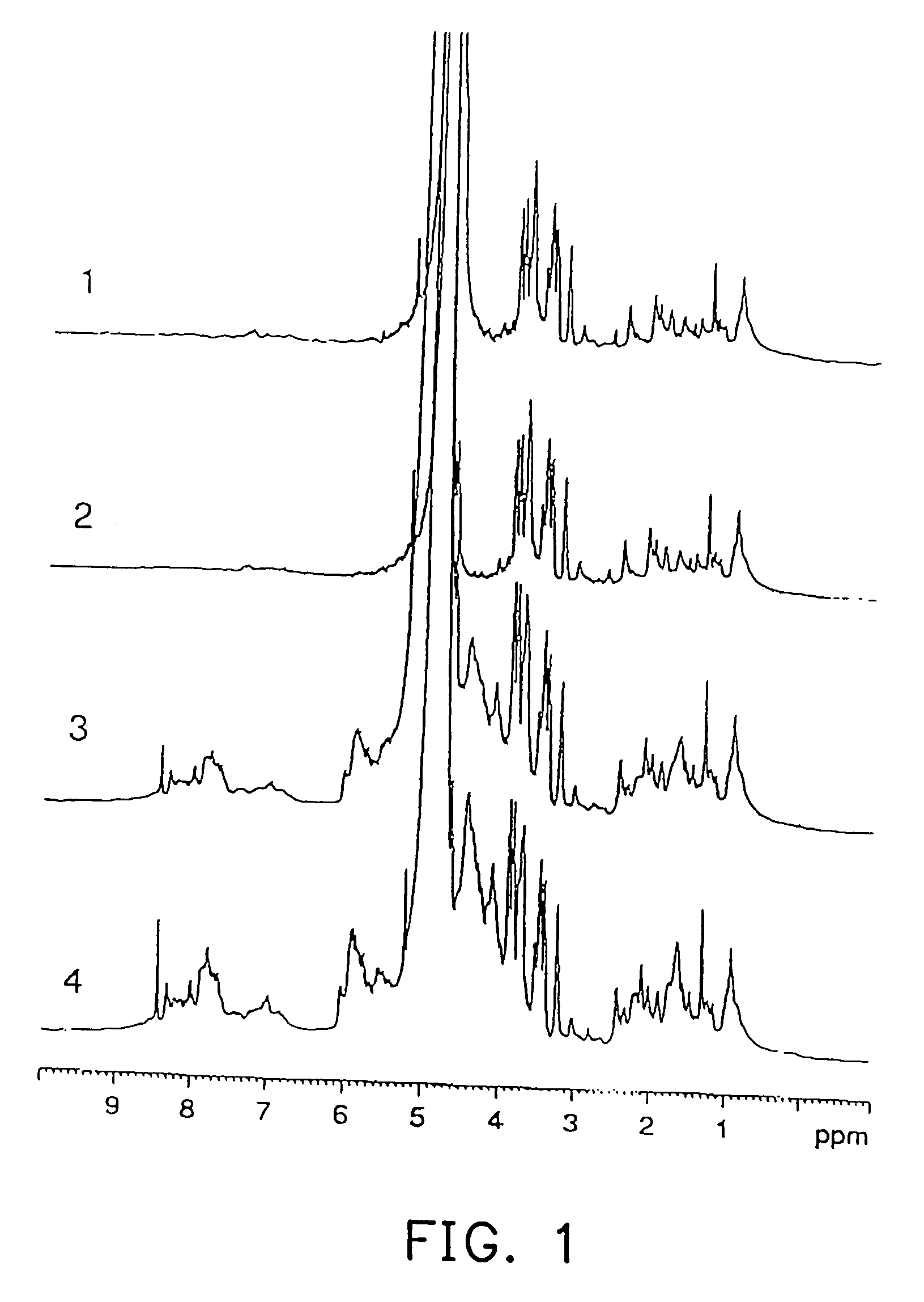
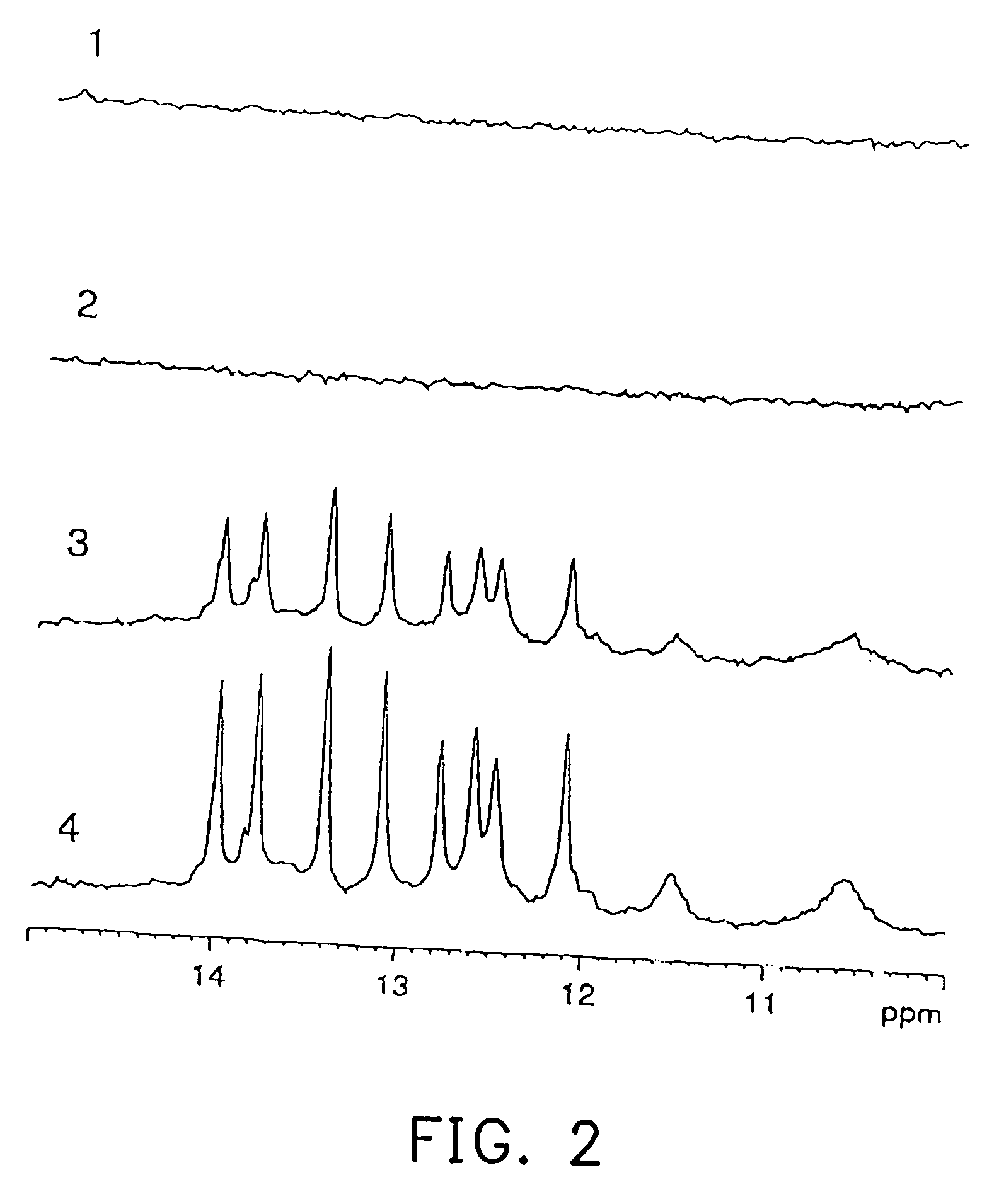
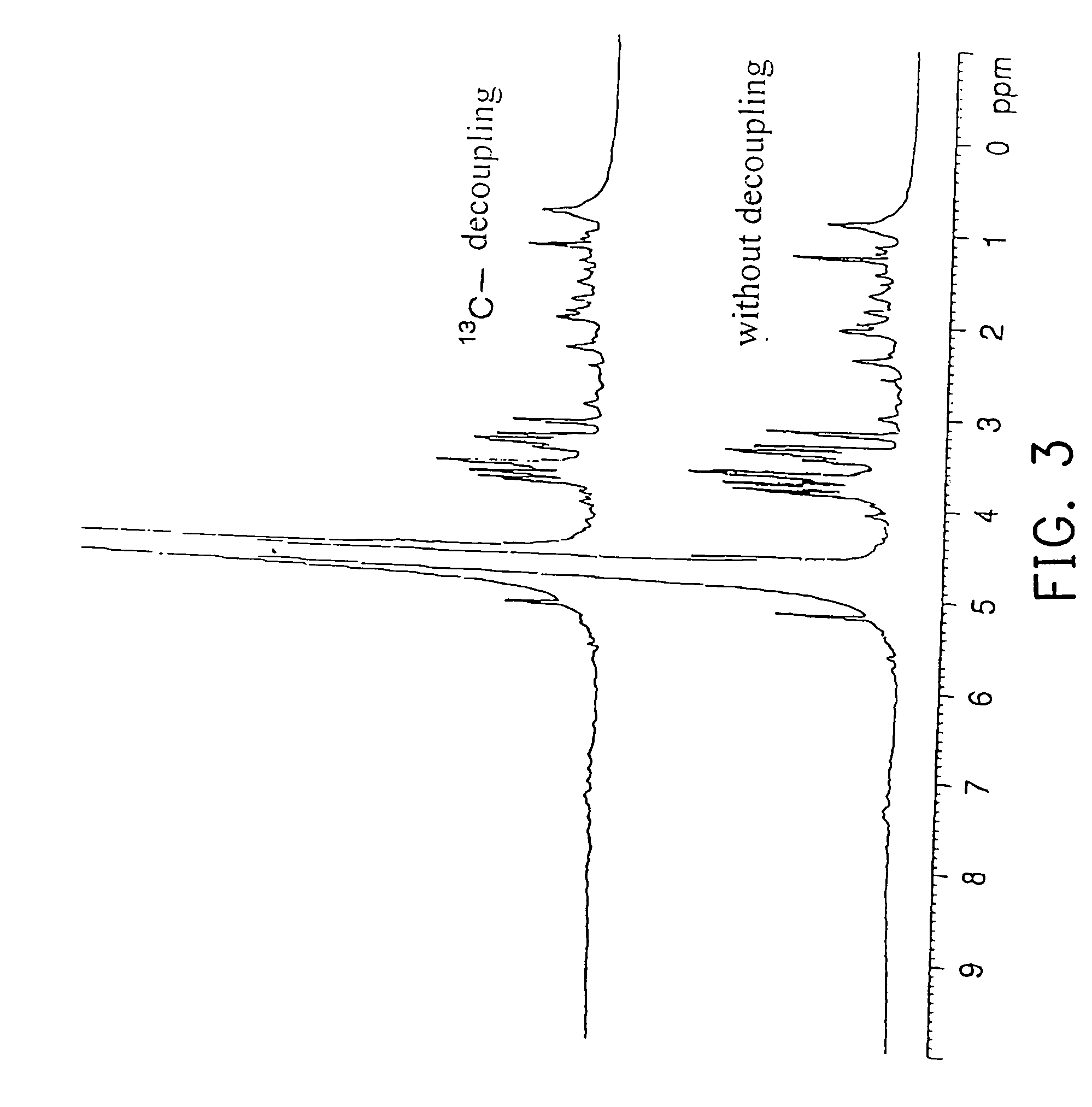
[0054] An experiment was carried out to confirm whether a RNA labeled with stable isotopes was detectable by NMR when the RNA was administered to mice.
Materials
[0055] Four female SPF / VAF mice (BALB / cAnNCr, 8 weeks old, No. 1 to No. 4) supplied by Charles River Japan, Inc. were used for the experiment.
[0056] A nucleotide labeled with 13C and 15N was synthesized by the method described in Japanese Patent Laid-open No. 1994-319581 and Japanese Patent Laid-open No. 1995-115987. An outline of this synthesizing method will be described as follows.
[0057] First, yeast cells of Candida utilis IFO-0369 were cultured in an inorganic medium using 13C-labeled acetic acid (13CH313COOH) as a carbon source and 15N-labeled ammonium chloride (15NH4Cl) as a nitrogen source, and then harvested. Cell walls were decomposed by a cell wall lytic enzyme, zymolyase (Kirin Breweries, Ltd.), after which the supernatant obtained by centrifugation was further centrifuged (100,000 g×3 hours) to obtain a ribo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Force | aaaaa | aaaaa |
| Structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com