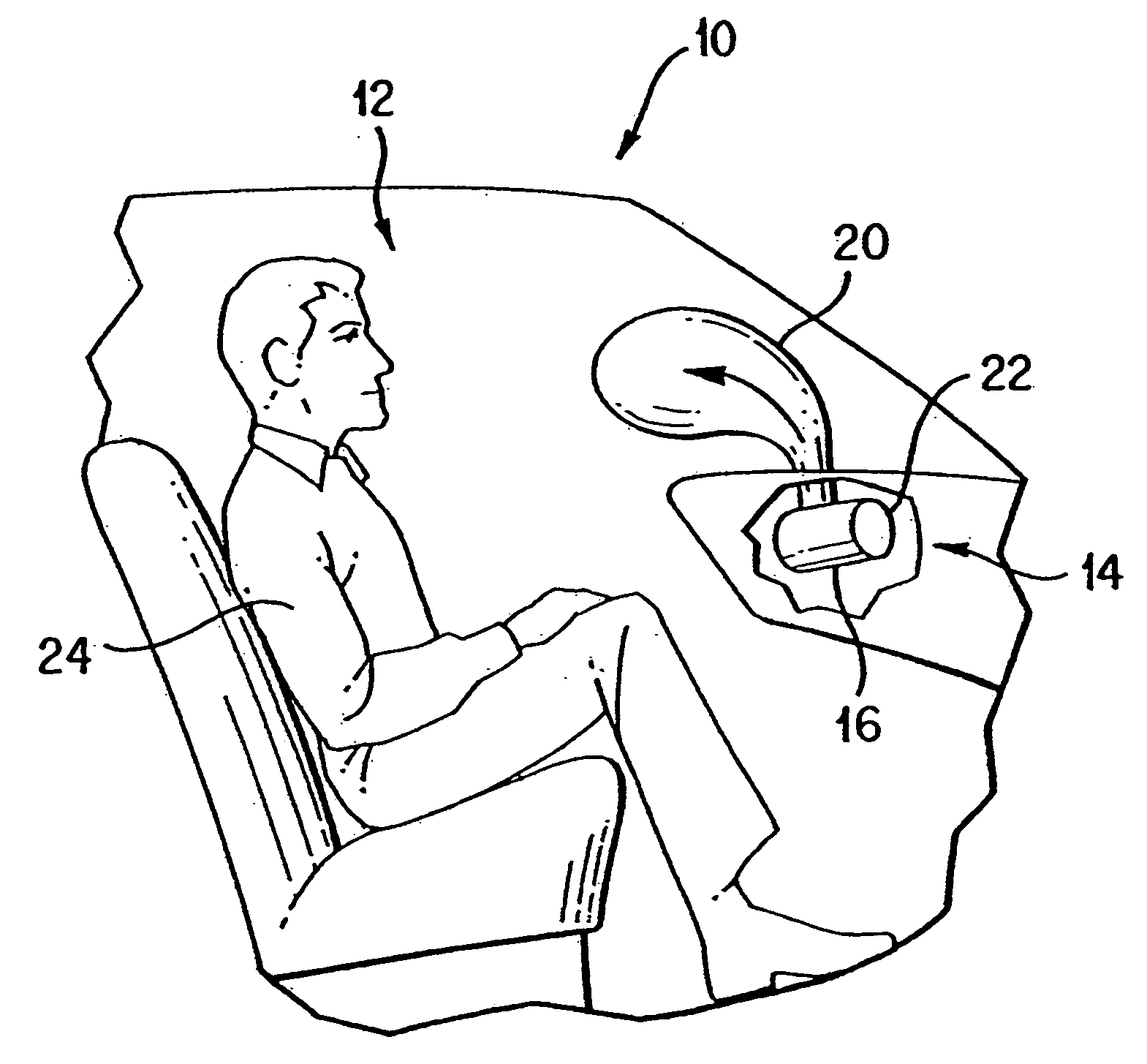
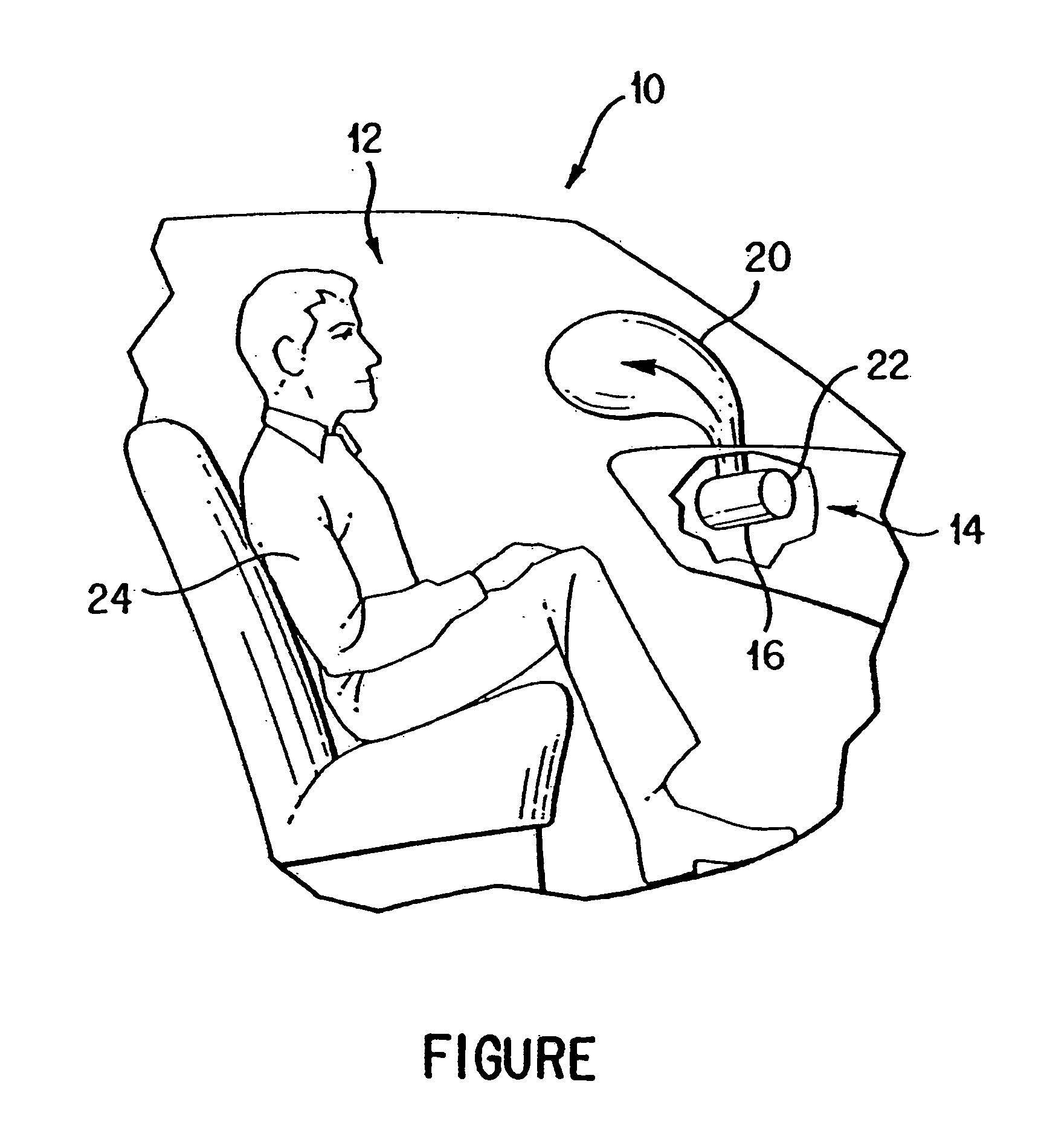
Gas generant materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0065] The same procedure as in Comparative Example 1 except that, 1) 5-aminotetrazole and bCN were reacted in water at 190° F. for 1 hour in order to make the 5-AT / bCN complex and 2) cupric oxide and diammonium bitetrazole were reacted in water at 190° F. for 1 hour in order to make the copper diammonium bitetrazole prior to the addition of the other ingredients.
TABLE 1CE 1CE 2CE 3Example 1GN50.3827.4924.6628.58bCN46.6214.3551.7221.36CuDABT——20.624.00bCuATN—55.16—43.36Al2O32.701.501.501.50SiO20.301.501.501.20
where,
[0066] GN=guanidine nitrate
[0067] bCN=basic copper nitrate
[0068] CuDABT=copper diammonium bitetrazole and
[0069] bCuATN=5-amino tetrazole substituted basic copper nitrate
[0070] The gas generant formulation of each of Comparative Examples 1-3 and Example 1 was then tested. The burn rate and combustion flame temperature (Tc) values identified in TABLE 2 below were obtained. In particular, the burn rate data was obtained by first pressing samples of the respective gas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com