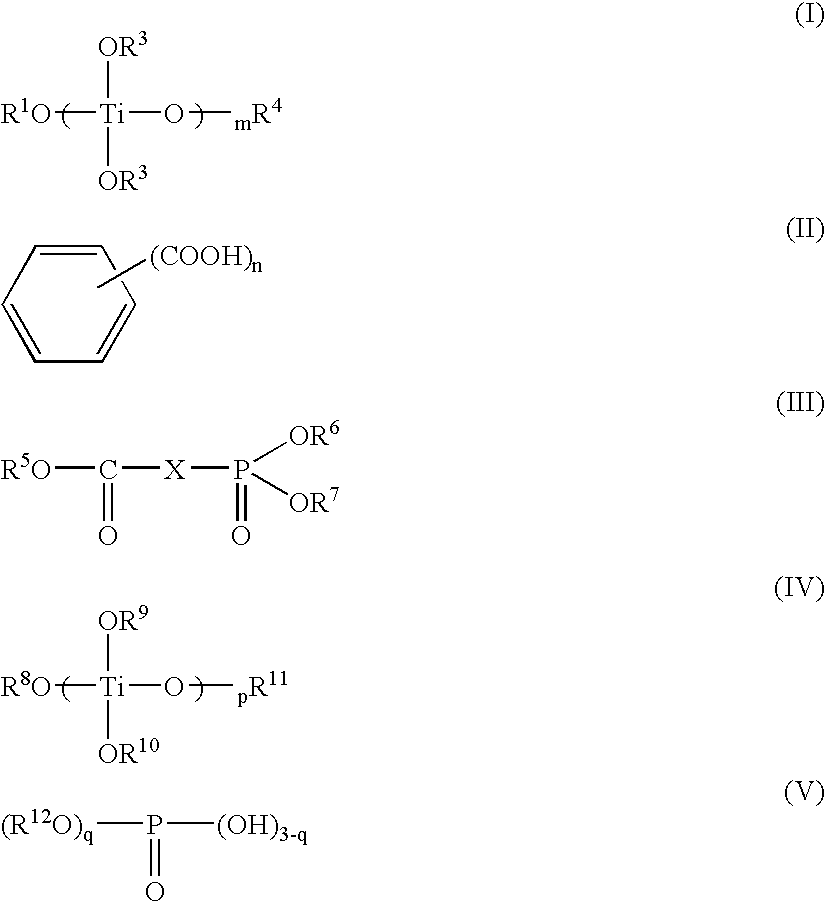Polyester fiber structures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0189] After charging 0.009 part of tetra-n-butyl titanate into a mixture of 100 parts of dimethyl terephthalate and 70 parts of ethylene glycol in a pressure reaction-capable stainless steel reactor, pressurization was conducted at 0.07 MPa for transesterification reaction while increasing the temperature from 140° C. to 240° C., and then 0.04 part of triethyl phosphonoacetate was added to terminate the transesterification reaction.
[0190] The reaction product was then transferred to a polymerization reactor, the temperature was raised to 290° C., and polycondensation reaction was conducted in a high vacuum of no greater than 26.67 Pa to obtain a (delustering agent-free) polyester with a limiting viscosity of 0.60, a diethylene glycol content of 1.5 wt % and a melting point of 254° C.
[0191] The obtained polyester was prepared into chips and dried by ordinary procedures. The dried chips were used for spinning, stretching, cutting, etc. according to ordinary methods to obtain polyes...
example 2
[0193] The same procedure was carried out as in Example 1, except that 0.016 part of titanium trimellitate synthesized by the method described in the reference example was used as the titanium compound. The results are shown in Table 1.
examples 3-5
, Comparative Examples 1-3
[0194] The same procedure was carried out as in Example 1, except for adding the titanium compounds and phosphorus compounds listed in Table 1 in the indicated amounts. The results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com