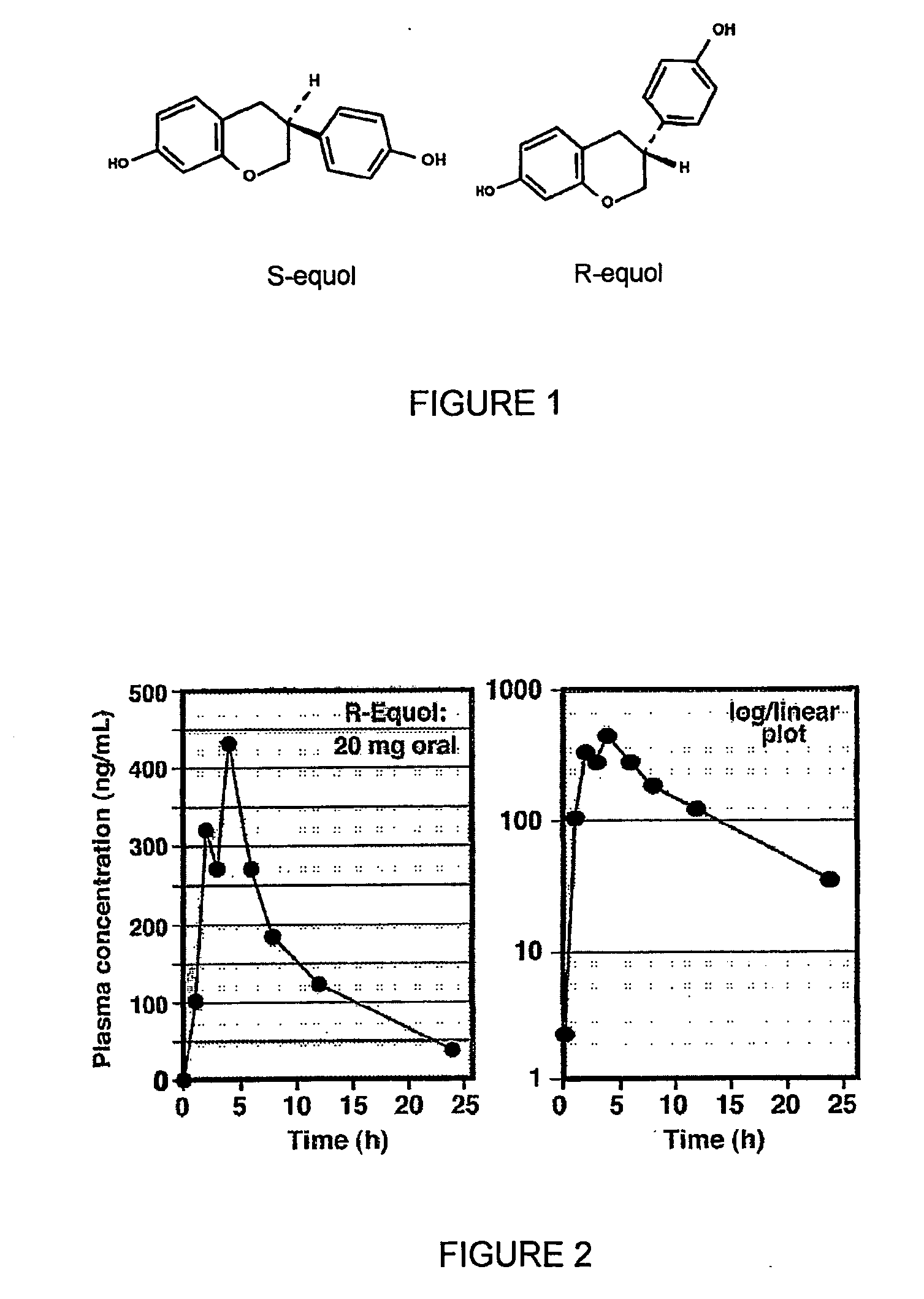
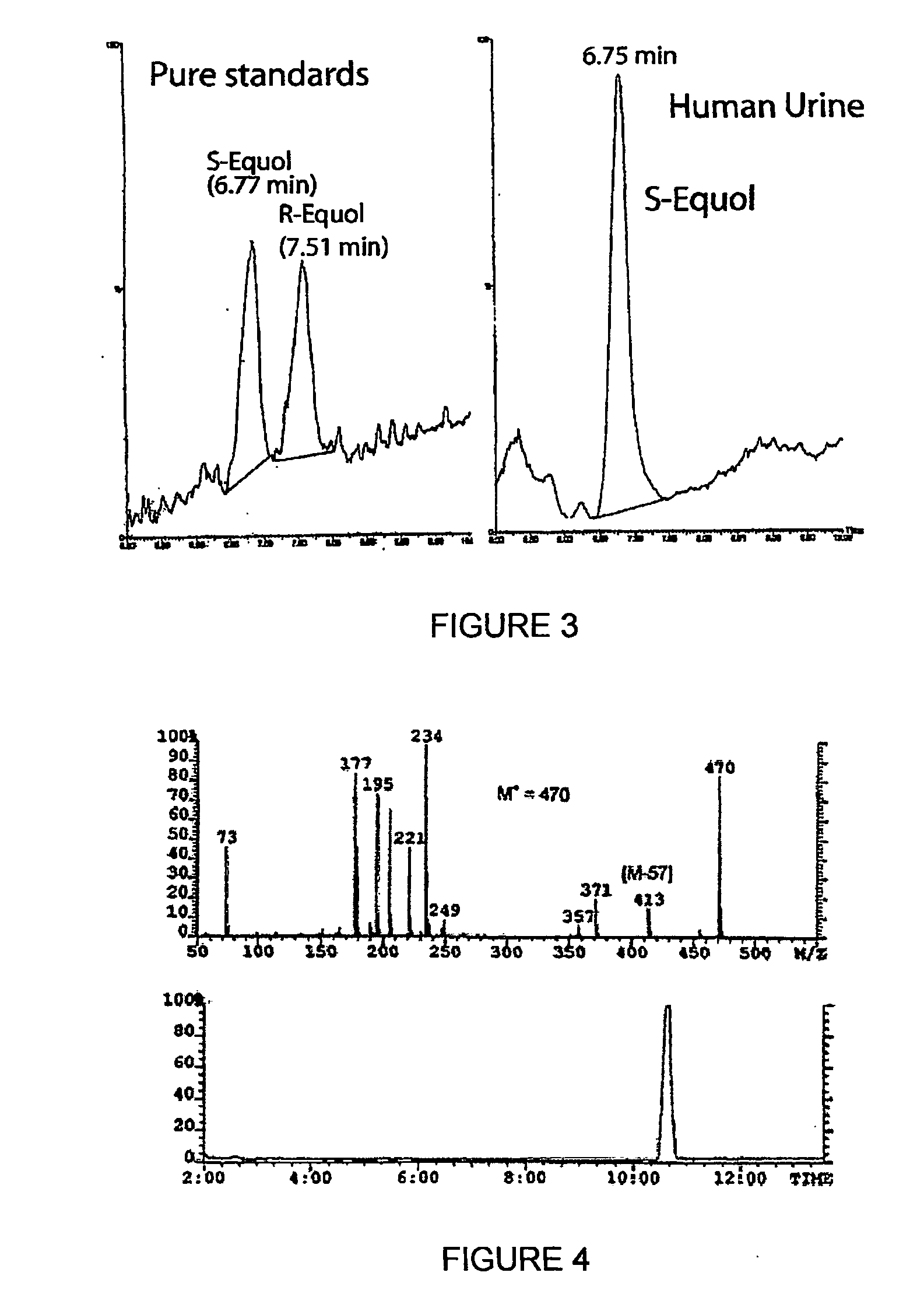
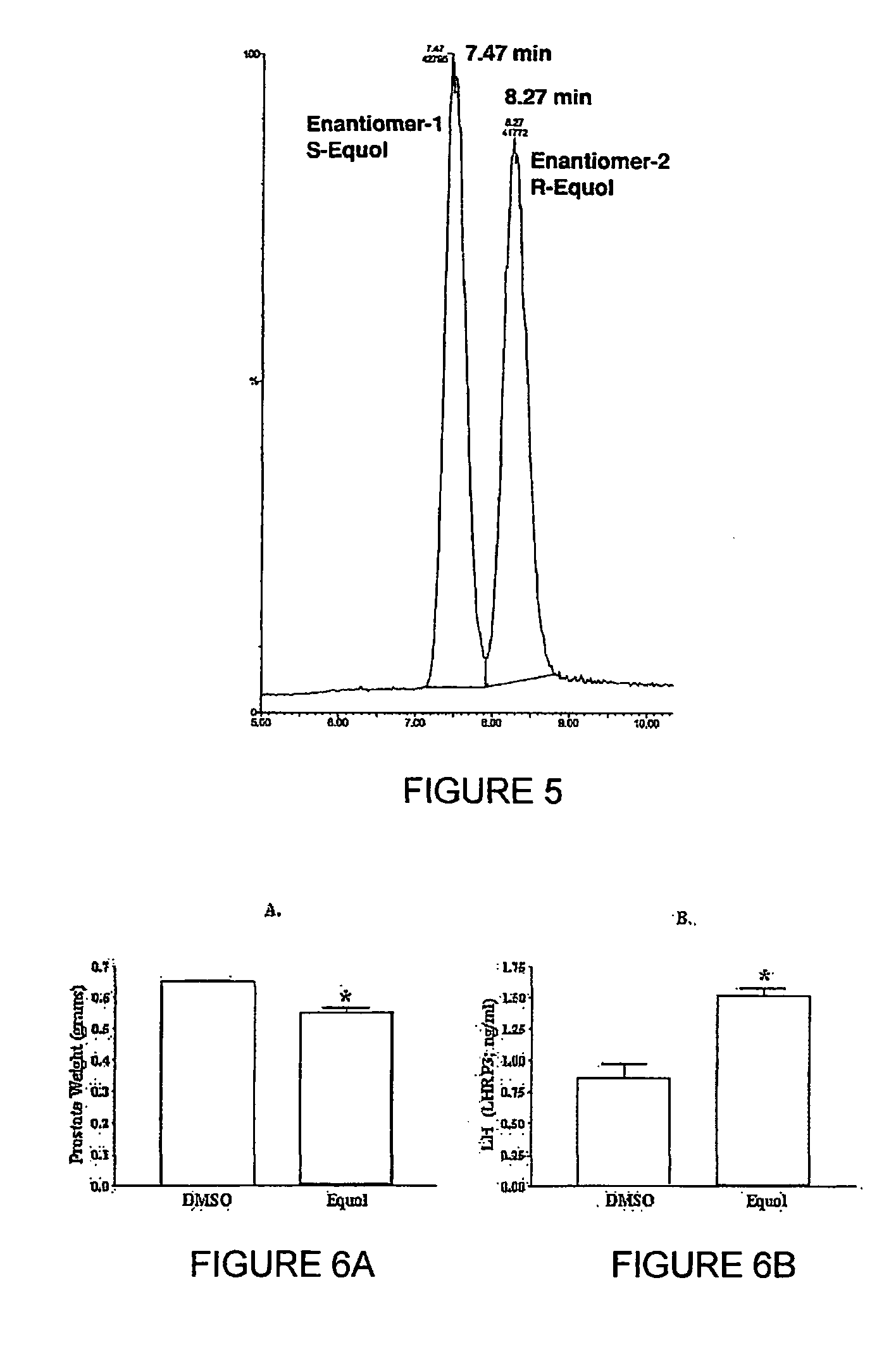
Use of equol for treating androgen mediated diseases
a technology of androgen mediated diseases and equol, which is applied in the direction of hair cosmetics, drug compositions, metabolic disorders, etc., can solve the problems of not testing for testosterone and unclear whether inhibition of 5-reductase will have a deleterious impact on the system, and achieve the effect of preventing dht binding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0159] This example demonstrates the in vivo effects of equol on prostate size and hormone secretion. Male Sprague-Dawley rats (400-500 grams) are obtained from Charles Rivers Laboratories (Wilmington, Mass., USA). Rats are caged in pairs and maintained on a 12-hour dark 12-hour light schedule (lights on at 0700 h) with ad libitum access to food and water.
[0160] One week following arrival, animals are given subcutaneous (sc) injections (1 / day for 4 days) of either dimethylsulfoxide (DMSO) (vehicle control) or racemic equol (0.25 mg / kg). Eighteen hours after the final injection, animals are killed via decapitation and trunk blood and prostate are collected for analysis.
[0161] A significant reduction in prostate weight is observed in intact males injected subcutaneously with equol in comparison to intact control males. Additionally, luteinizing hormone (LH) in these same intact males is significantly increased in equol compared to control treated males. These finding are shown in FI...
example 2
[0162] In addition to equol's effects on prostate racemic equol blocks the effects of DHT in other tissues, and decreases body weight. One week following arrival, intact males are given subcutaneous injections of either DMSO (control) or equol (0.5 mg / kg) once / day for 7 days. Following treatments animals are weighed and then killed via decapitation and tissue collection (prostate, testes, epididymis, and pituitary).
[0163] A significant weight decrease in the DHT-sensitive epididymis is observed in racemic equol-treated males compared to controls. However, racemic equol did not affect testes weight or pituitary weight. Body weight during this relatively brief treatment period does not differ significantly between racemic equol and control treatments. These results are presented in Table 2.
TABLE 2Tissue weights from intact males given subcutaneouslyinjections of either DMSO (control) or racemic equol.Prostate (g)Epididymis (g)Testes (g)Pituitary (g)Body (g)DMSO (Control)0.58 (±0.05...
example 3
[0164] Adult male Sprague-Dawley rats are randomly assigned to three groups and receive daily injections of either DMSO, a racemic mixture of equol at 0.250 mg / kg / day), R-equol at 0.250 mg / kg / day, or S-equol at 0.250 mg / kg / day. The total volume of each injection is 0.3 cc, administered sc at the nape of the rat's neck After seven consecutive days of treatment, the rats are killed and the body weight gain during the injection period is determined. Rats injected with R-equol have a significant decrease in body weight gain compared to control rats, as shown in Table 3.
TABLE 3Body Weight Gained in Male Sprague-Dawley Rats Treated with Equol.Treatment GroupProstate (g)Epididymis (g)Body Wt Gain (g)Control0.38 (±0.01)0.96 (±0.03)59.4 (±3.4)Racemic Equol (0.25 mg / kg)0.35 (±0.02)0.89 (±.03) 56.1 (±3.7)R-equol (0.25 mg / kg) 0.31 (±0.02)* 0.85 (±0.04)* 45.6 (±5.5)*S-equol (0.25 mg / kg)0.35 (±0.03)0.86 (±0.05)56.1 (±3.1)
*Significant reduction compared to control
[0165] Testis and pituitary gla...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| pressures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com