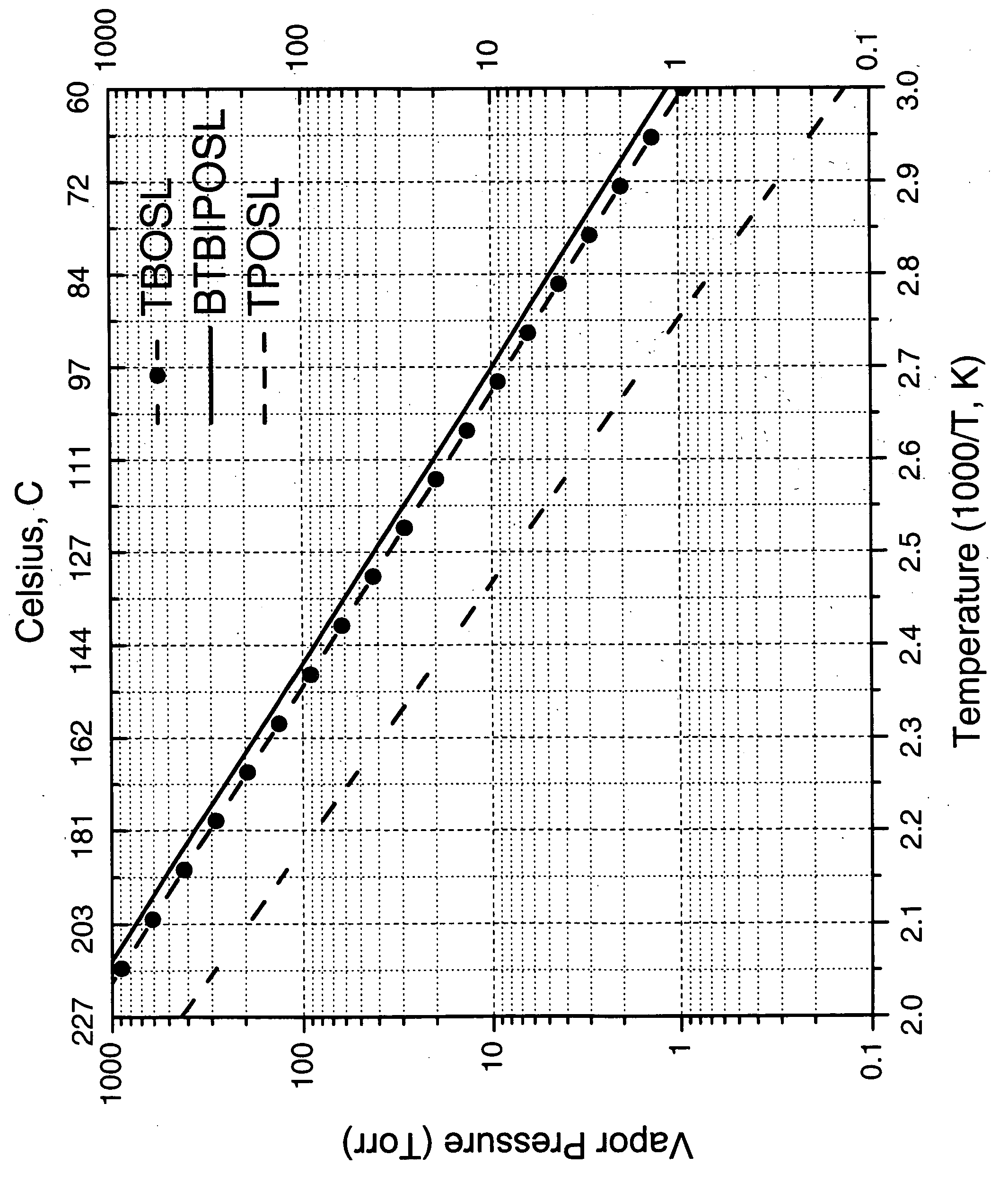
Precursors for silica or metal silicate films
a technology of metal silicate films and precursors, which is applied in the direction of silicates, silicon compounds, organic chemistry, etc., can solve the problems of undesirable physical properties of alkoxysilanols that have been presently contemplated for manufacturing silica-metal films, and inadequate traditional silica dielectric insulating materials between those component devices and circuits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of bis(iso-propoxy)(tert-pentoxy)silanol
[0023] 20 g (0.118 mol) of SiCl4 was loaded in a 1000 ml three-neck flask with 500 ml hexanes. The flask was cooled down to −40° C. with a cold bath containing dry ice and iso-propanol. 18 ml (0.237 mol) of iso-propanol and 11.3 ml (0.118 mol) of tert-pentanol were added slowly via addition funnel. The temperature was kept at less than −20° C. 30 ml of pyridine was added to the flask slow to generate a lot of white precipitate which is pyridine.HCl. The cold bath was removed and the flask was stirred for 3 hours. Gas chromatograph / mass spectrometer (GC / MS) measurement of the reaction mixture indicated formation of bis(iso-propoxy)(tert-pentoxy)chlorosilane, plus other products. Filtration and removal of solvents resulted in about 23 g of white slurry. The white slurry was dropwise added to a solution containing 40 ml of ether, 15 ml of water, and 15 ml of pyridine at a temperatue below −20° C. until completion of the addition. The r...
example 2
Synthesis of bis(iso-propoxy)(tert-butoxy)silanol
[0024] 20 g (0.118 mol) of SiCl4 was loaded in a 1000 ml three-neck flask with 500 ml hexane. The flask was cooled down to −40° C. with a cold bath containing dry ice and iso-propanol. 11.3 ml (0.118 mol) of tert-butanol was added slowly via addition funnel. The temperature was kept at less than −20° C. 10 ml of pyridine was added to the flask slowly to generate a lot of white precipitate, which is pyridine.HCl. The cold bath was removed and the flask was stirred for 3 hours. A mixture of 18 ml iso-propanol and 20 ml pyridine were added slowly to the resulting white slurry. The reaction mixture was stirred overnight. GC / MS measurement of the reaction mixture indicated formation of bis(iso-propoxy)(tert-butoxy)chlorosilane and tris(iso-propoxy)(tert-butoxy)silane. Filtration and removal of solvents resulted in white slurry. The white slurry was dropwise added to a solution containing 50 ml of ether, 50 ml of water, and 15 ml of pyridi...
example 3
Synthesis of bis(tert-butoxy)(iso-propoxy)silanol
[0025] 20 g (0.118 mol) of SiCl4 was loaded in a 1000 ml three-neck flask with 400 ml hexane. The flask was cooled down to −40° C. with a cold bath containing dry ice and iso-propanol. 22.6 ml (0.236 mol) of tert-butanol was added slowly via addition funnel. The temperature was kept at less than −20° C. 20 ml of pyridine was added to the flask slowly to generate a lot of white precipitate, which is pyridine.HCl. The cold bath was removed and the flask was stirred overnight after removal of the cold bath. Filtration was applied to give a liquid to which 9 ml (0.118 mol) of iso-propanol was added at temperature below 40° C. 10 ml of pyridine was added slowly to the resulting white slurry. The reaction mixture was stirred for 5 hours after removal of the cold bath. Filtration and removal of solvents resulted in white slurry. The white slurry was dropwise added to a solution containing 50 ml of ether, 50 ml of water, and 15 ml of pyridin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| vapor pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com