Method, instruments, and kit for autologous transplantation
a technology of autologous and autologous fusion, which is applied in the field of autologous fusion methods, instruments, kits, etc., and can solve the problems of matrix substances such as proteoglycans being lost, affecting the healing effect, and eventually displaced completely
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
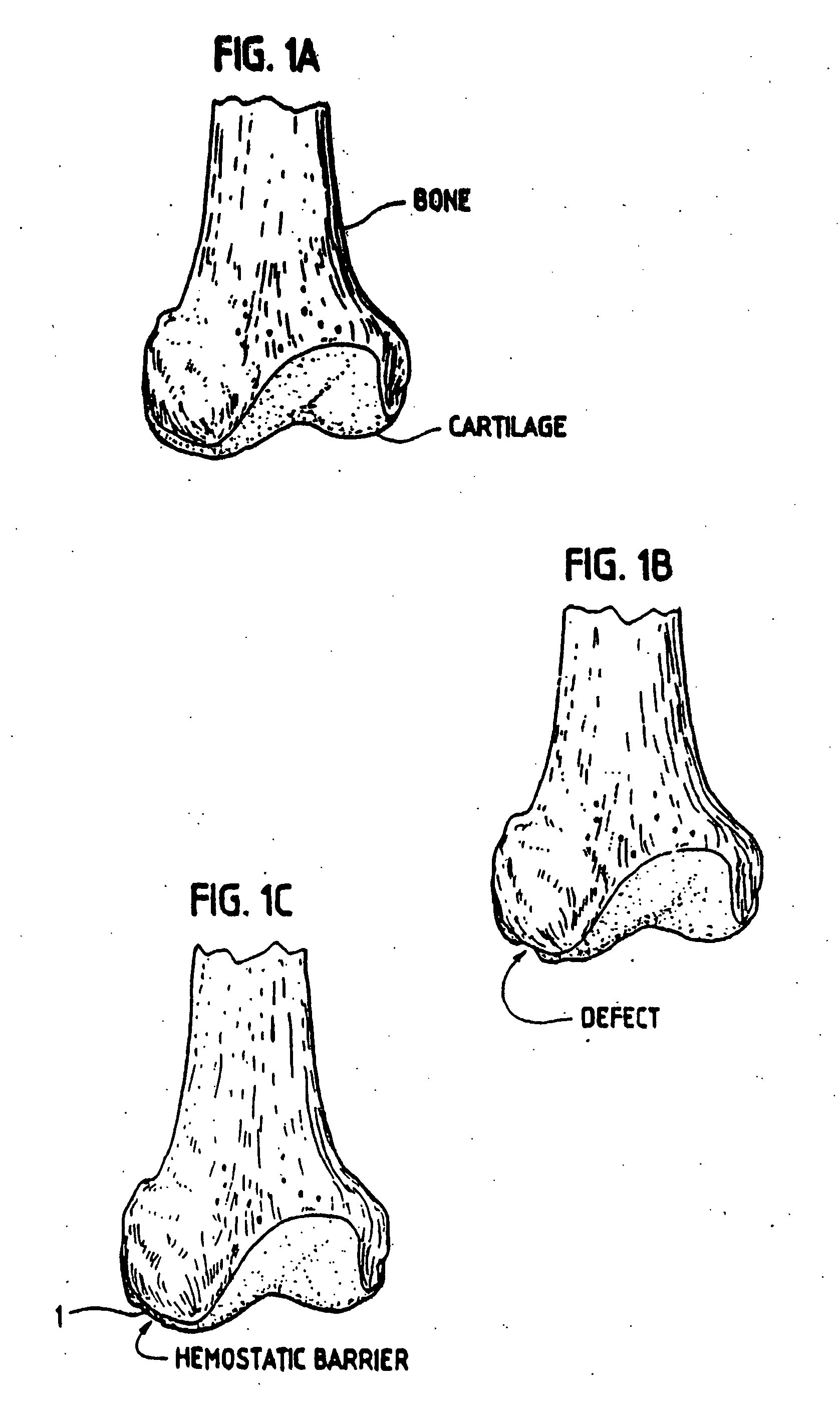
[0045] In order for the Surgicel® to be used according to the invention for preventing development of blood vessels into autologous implanted cartilage or chondrocytes, Surgicel® was first treated with a fixative, such as glutaric aldehyde. Briefly, Surgicel® was treated with 0.6% glutaric aldehyde for 1 minute, followed by several washings to eliminate glutaric aldehyde residues that may otherwise be toxic to tissue. Alternatively, the Surgicel® was treated with the fibrin adhesive called Tisseel® prior to treatment with glutaric aldehyde as described in Example 2. It was found that the Surgicel® fixated for instance with a fixative such as glutaric aldehyde, washed with sterile physiological saline (0.9%) and stored in refrigerator, does not dissolve for 1 to 2 months. Generally, Surgicel® is resorbed in a period between 7 and 14 days. This time would be too short, because a longer time is needed in preventing the development of blood vessels or vascularization as such from the bo...
example 2
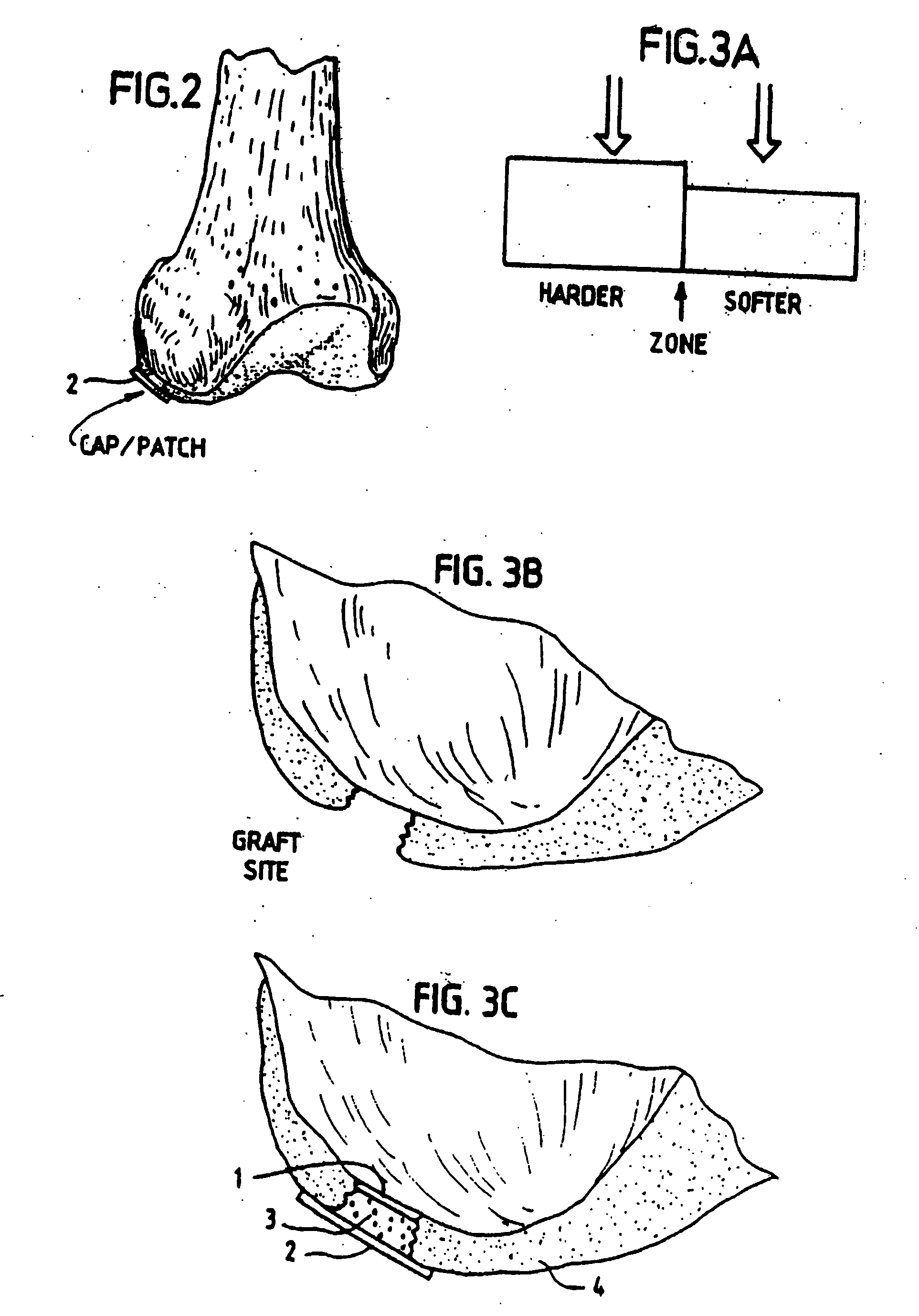
[0046] The Surgicel® was also coated with an organic glue, in this example the glue used was Tisseel® but others can also be used. This product, together with the Surgicel® produces a useable barrier for the particular purpose of the invention. Any other hemostat or vascular inhibiting barrier could be used. The Tisseel® was mixed as described below. The Surgicel® was then coated with Tisseel® by spraying the Surgicel® material on both sides until soaked. The Tisseel® (fibrin glue) was then allowed to solidify at room temperature. Immediately prior to completed solidification, the coated Surgicel® was then placed in 0.6% glutaric aldehyde for 1 minute and then washed with sterile physiological (0.9%) saline. The pH was then adjusted by PBS and / or with NaOH until pH was stable at 7.2 to 7.4. Afterwards the thus treated Surgicel® was then washed in tissue culture medium such as minimum essential medium / F12 with 15 mM Hepes buffer.
[0047] As mentioned in this example we have used Tisse...
example 3
[0055] Chondrocytes were grown in minimal essential culture medium containing HAM F12 and 15 mM Hepes buffer and 5 to 7.5% autologous serum in a CO2 incubator at 37° C. and handled in a Class 100 laboratory at Verigen Europe A / S, Symbion Science Park, Copenhagen, Denmark. Other compositions of culture medium may be used for culturing the chondrocytes. The cells were trypsinized using trypsin EDTA for 5 to 10 minutes and counted using Trypan Blue viability staining in a Burker-Turk chamber. The cell count was adjusted to 7.5×105 cells per ml. One NUNCLON™ plate was uncovered in the Class 100 laboratory.
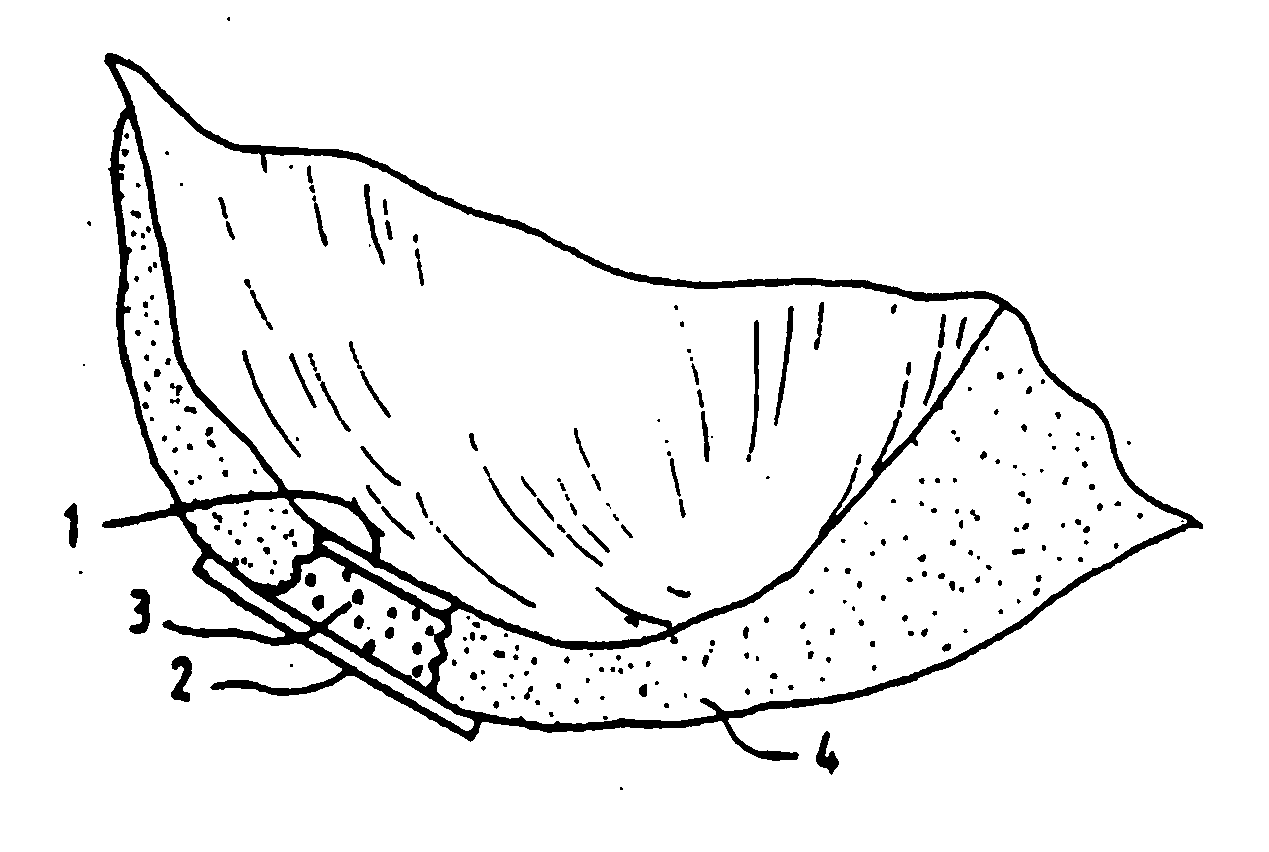
[0056] The Surgicel® hemostatic barrier was cut to a suitable size fitting into the bottom of the well in the NUNCLON™ tissue culture tray. In this case a circle, of a size of approximately 4 cm (but could be of any possible size) and placed under aseptic conditions on the bottom in well in a NUNCLON™ Delta 6-well sterile disposable plate for cell research work (NUNC, InterMed, Roskil...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com