Peptide factor
a technology of peptides and peptides, applied in the field of peptide factors, can solve problems such as serious side effects of steroids, and achieve the effect of increasing the half life of oxidised thymosin 4
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Steroid Treated Monocyte Supernatant (STMS) and Partially Purified STMS Peptide Factor
[0103] Steroid treated monocyte supernatant (STMS) was obtained by the culture of human monocytes which had been plated out in Hams F-10 medium at a concentration of 5×107 cells per ml in the presence of heat-inactivated 10% foetal calf serum (FCS) for 60 mins, rinsed with Phosphate buffered saline (PBS) and then cultured in the absence of FCS for 24 hours in the presence of 10−6M dexamethasone.
STMS Peptide Factor Preparation
[0104] STMS was obtained essentially as described by Chettibi et al. by the culture of human monocytes in Hams F-10 medium with 10% foetal calf serum (FCS) at a concentration of approximately 5×107 cells per ml for 60 minutes, rinsed with PBS, and then cultured without FCS for 24 hours in the presence of 10−6M dexamethasone. Parallel cultures in which dexamethasone was omitted were used to prepare control monocyte supernatant (CMS). Purification of STMS pepti...
example 2
Biological Studies of STMS Peptide Factor
[0108] The biological interest in STMS Peptide Factor lies in its potential role as a mediator of some or all of the anti-inflammatory effects of glucocorticoids. Many preliminary observations using the supernatant as opposed to the peptide factor seemed to support this role, but others, such as the phenomenon of dispersive locomotion, were not obvious anti-inflammatory responses. However, lowered adhesiveness, which appears to be one of the underlying causes of dispersive locomotion, has clear anti-inflammatory implications.
Characteristics of Neutrophil Locomotion
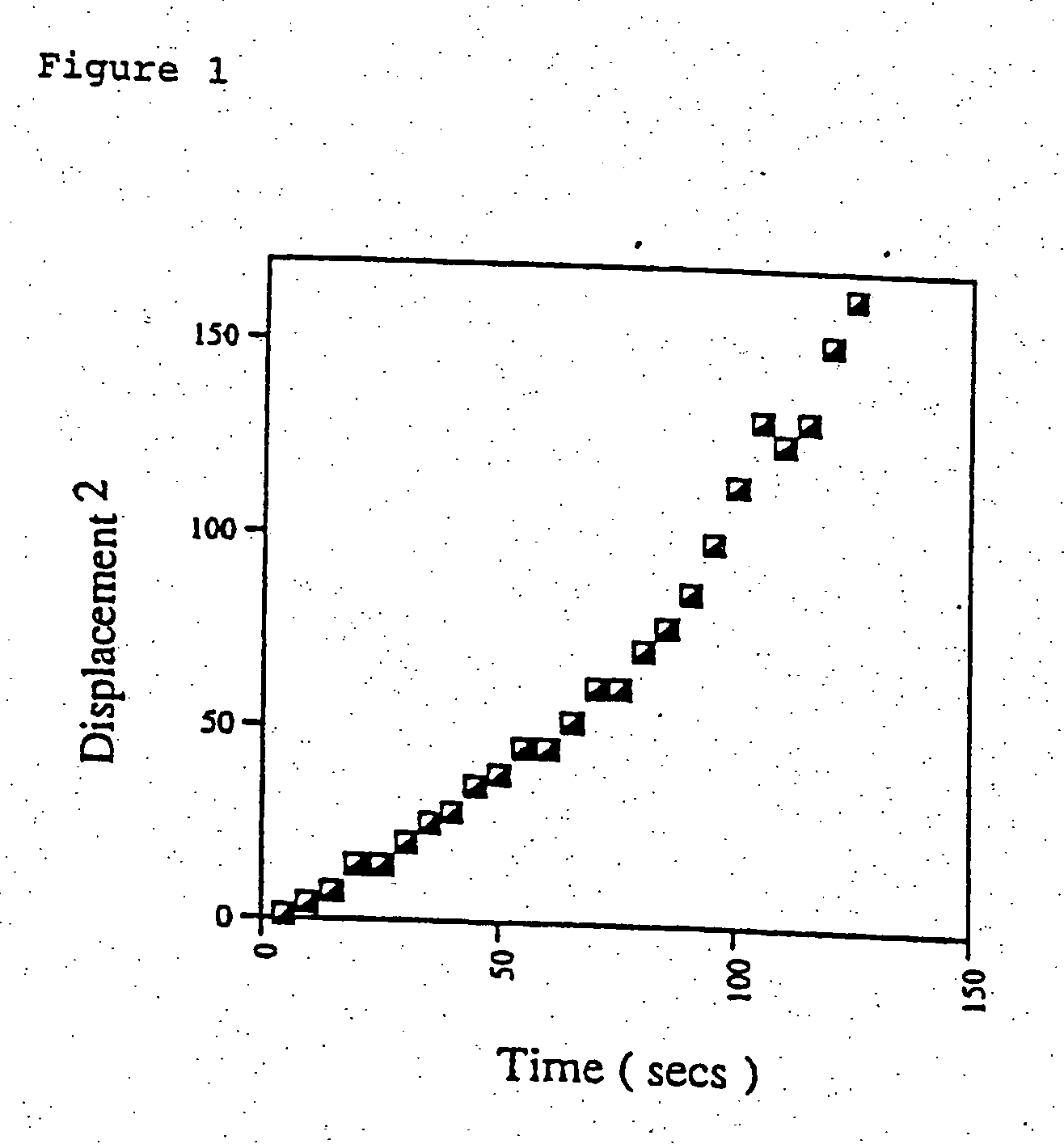
[0109] Agonists were used at concentrations which caused similar, sub-maximal stimulation of basal motility. Previous studies of crude and partially purified STMS peptide factor showed that it stimulated neutrophils to undergo highly dispersive locomotion in a uniform concentration gradient. This was in marked contrast to responses to other agents and in particular to fMLP where...
example 3
Detailed Biological Studies of STMS and Peptide Factor
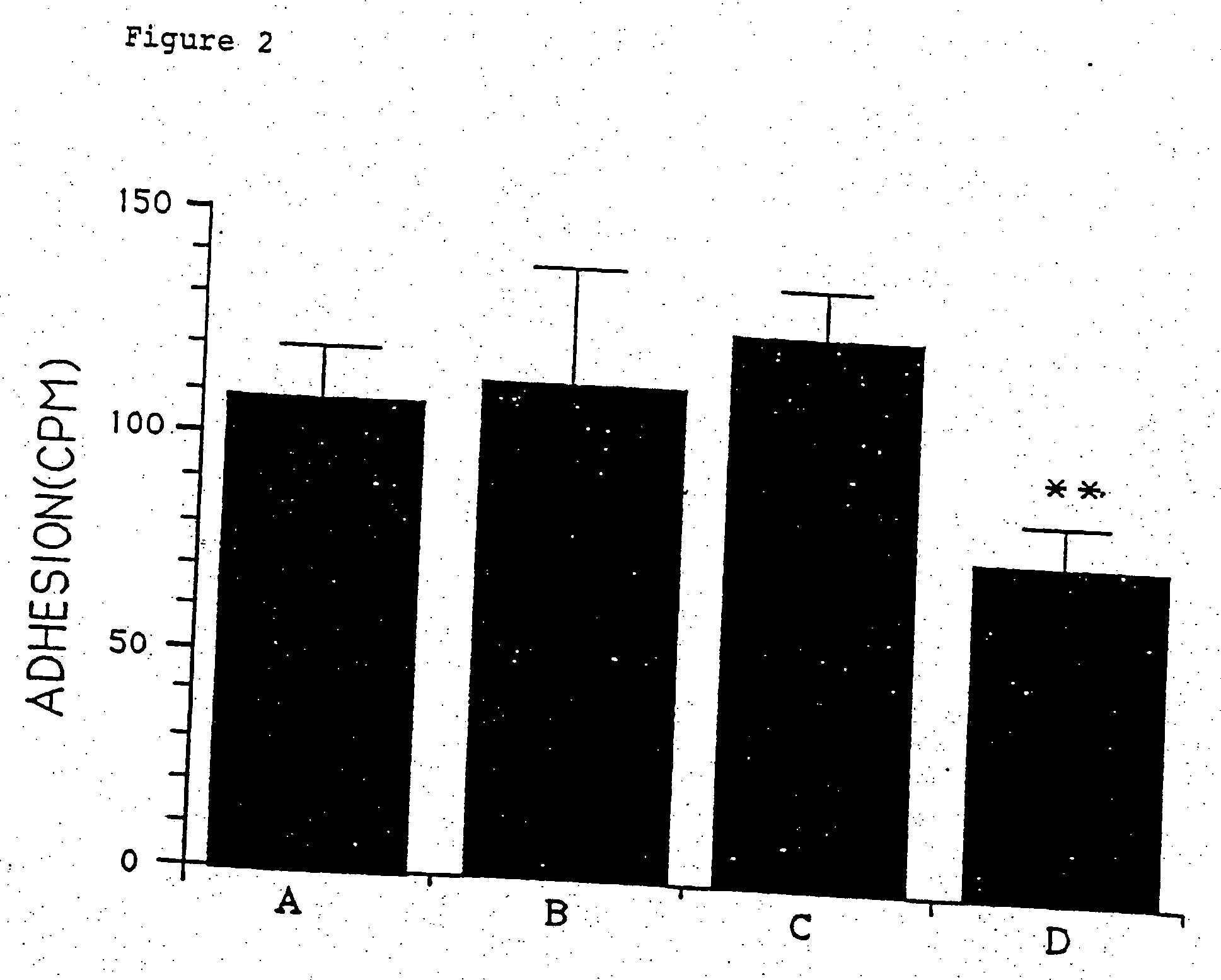
[0120] Neutrophil Adhesion to Bovine Aorta Endothelial Cells
[0121] The previous data showing that STMS diminished neutrophil adhesion to protein-coated glass [Chettibi et al., 1993] was extended using the more physiological substrate of bovine aorta endothelial cells (FIG. 2).
[0122] Chemotaxis
[0123] Preliminary observations of neutrophil chemotaxis using the modified Boyden chamber revealed a striking contract between the response to STMS and fMLP. Neutrophils showed massive invasion of the filter when fMLP was present in the lower chamber, but gave no such response to STMS. However when the filters were examined by the leading front method it became clear that a few STMS-treated cells were able to invade successfully, in keeping with the predictions for persistent locomotion. These observations suggested that a more suitable analysis would be to measure total invasion, or to determine the number of cells present midway betwe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com