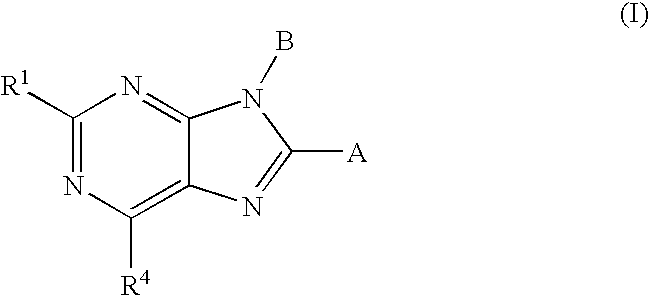
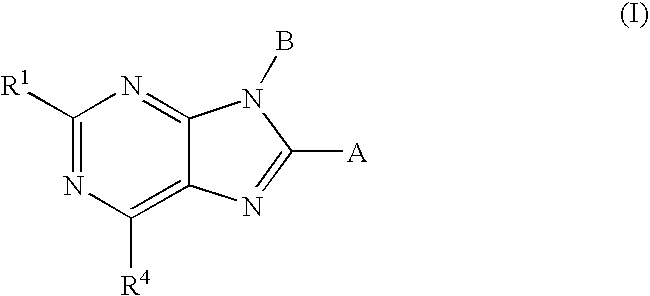
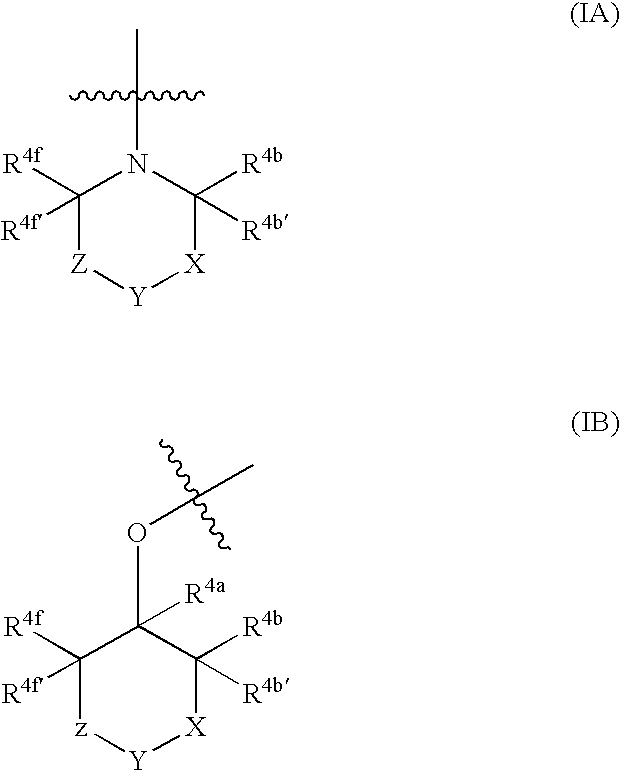
Cannabinoid Receptor Ligands and Uses Thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 9-(4-Chlorophenyl)-8-(2,4-dichlorophenyl)-6-isopropoxy-9H-purine (IA-1)
[0265]
[0266] Sodium (7 mg, 0.3 mmol) was dissolved in isopropanol (1 ml) and to it was added 6-chloro-9-(4-chlorophenyl)-8-(2,4-dichlorophenyl)-9H-purine I-(1A-1)d (30 mg, 0.07 mmol). After stirring at room temperature overnight, the reaction mixture was evaporated to dryness and extracted into ethyl acetate from saturated aqueous NaHCO3 solution. The organic layers were combined, dried (Na2SO4), filtered, and concentrated to dryness. The crude residue was purified on a TLC preparative plate using 4% methanol / methylene chloride as the solvent to yield title compound 1A-1. A solution of the material in methylene chloride was treated with excess 1 N HCl in diethyl ether, stirred, evaporated to dryness, and then triturated in diethyl ether to afford the hydrochloride salt of compound 1A-1 (8 mg, 26%): +ESI MS (M+1) 433.4; 1H NMR (400 MHz, CD3OD) δ 8.50 (s, 1H), 7.64 (d, J=8.3 Hz, 1H), 7.55 (d, J=1.7 ...
example 2
Preparation of 6-tert-Butoxy-9-(4-chlorophenyl)-8-(2,4-dichlorophenyl)-9H-purine (2A-1)
[0268]
[0269] 6-Chloro-9-(4-chlorophenyl)-8-(2,4-dichlorophenyl)-9H-purine I-(1A-1)d, potassium tert-butoxide (20 mg, 0.15 mmol) and tetrahydrofuran (1 ml) were combined and stirred overnight at ambient temperature. The reaction mixture was concentrated to dryness and the residue was extracted into ethyl acetate from saturated aqueous NaHCO3 solution. The organic layers were combined, dried (Na2SO4), filtered, and evaporated to dryness. The crude product was purified by chromatography on a preparative TLC plate using 4% methanol / methylene chloride as the solvent to give title compound 2A-1 as a yellow oil: +ESI MS (M+1) 447.5; 1H NMR (400 MHz, CD3OD) δ 8.48 (s, 1H), 7.64 (d, J=8.3 Hz, 1H), 7.55 (d, J=2.1 Hz, 1H), 7.49-7.44 (m, 3H), 7.34 (d, J=9.1 Hz, 2H), 1.77 (s, 9H).
example 3
Preparation of 6-(1-Benzhydrylazetidin-3-yloxy)-9-(4-chlorophenyl)-8-(2,4-dichlorophenyl)-9H-purine (3A-1)
[0270]
[0271] To a tetrahydrofuran (1 ml) solution of 6-chloro-9-(4-chlorophenyl)-8-(2,4-dichlorophenyl)-9H-purine I-(1A-1)d (30 mg, 0.073 mmol) and 1-benzhydrylazetidin-3-ol (53 mg, 0.22 mmol) was added potassium tert-butoxide (24 mg, 0.22 mmol). The combined reagents were stirred overnight at ambient temperature. The reaction mixture was concentrated to dryness and the residue was extracted into ethyl acetate from saturated NaHCO3 solution. The organic layers were combined, dried (Na2SO4), filtered, and evaporated to dryness. The crude product was purified by chromatography on a preparative TLC plate using 4% methanol / methylene chloride as the solvent to give title compound 3A-1 as a yellow oil. A solution of the material in methylene chloride was treated with excess 1 N HCl in diethyl ether, stirred, evaporated to dryness, and then triturated in diethyl ether to afford the hy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electrical conductance | aaaaa | aaaaa |
| Electrical conductance | aaaaa | aaaaa |
| Electrical conductance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com