Process for corrosion control in boilers
a technology of corrosion control and boiler, which is applied in the direction of instruments, combustion types, and the nature of treatment water, etc., can solve the problems of insufficient cost-effectiveness of replacement alloys, the inability to replace superheater pendants, and the corrosion of high temperature surfaces of chloride-induced high temperature surfaces in waste to energy boilers. the effect of operating load
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
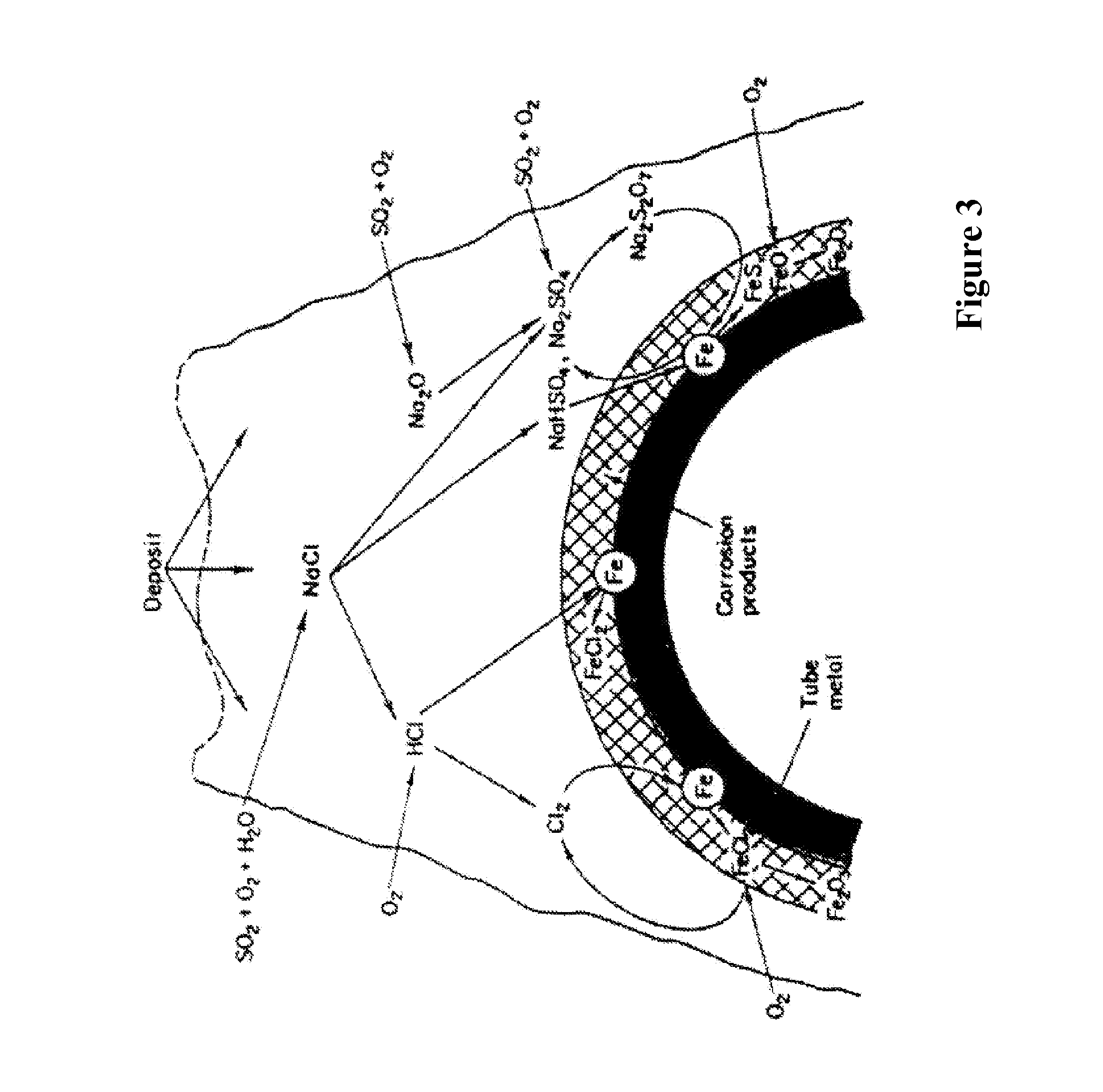
[0040] The invention provides processes for monitoring corrosion and for controlling corrosion, which are described below with reference to exemplary embodiments. Because the monitor and the processes are especially useful in the control of chloride corrosion in waste to energy boilers, they will be described in this context while those skilled in the art will see the application of the invention to other environments.
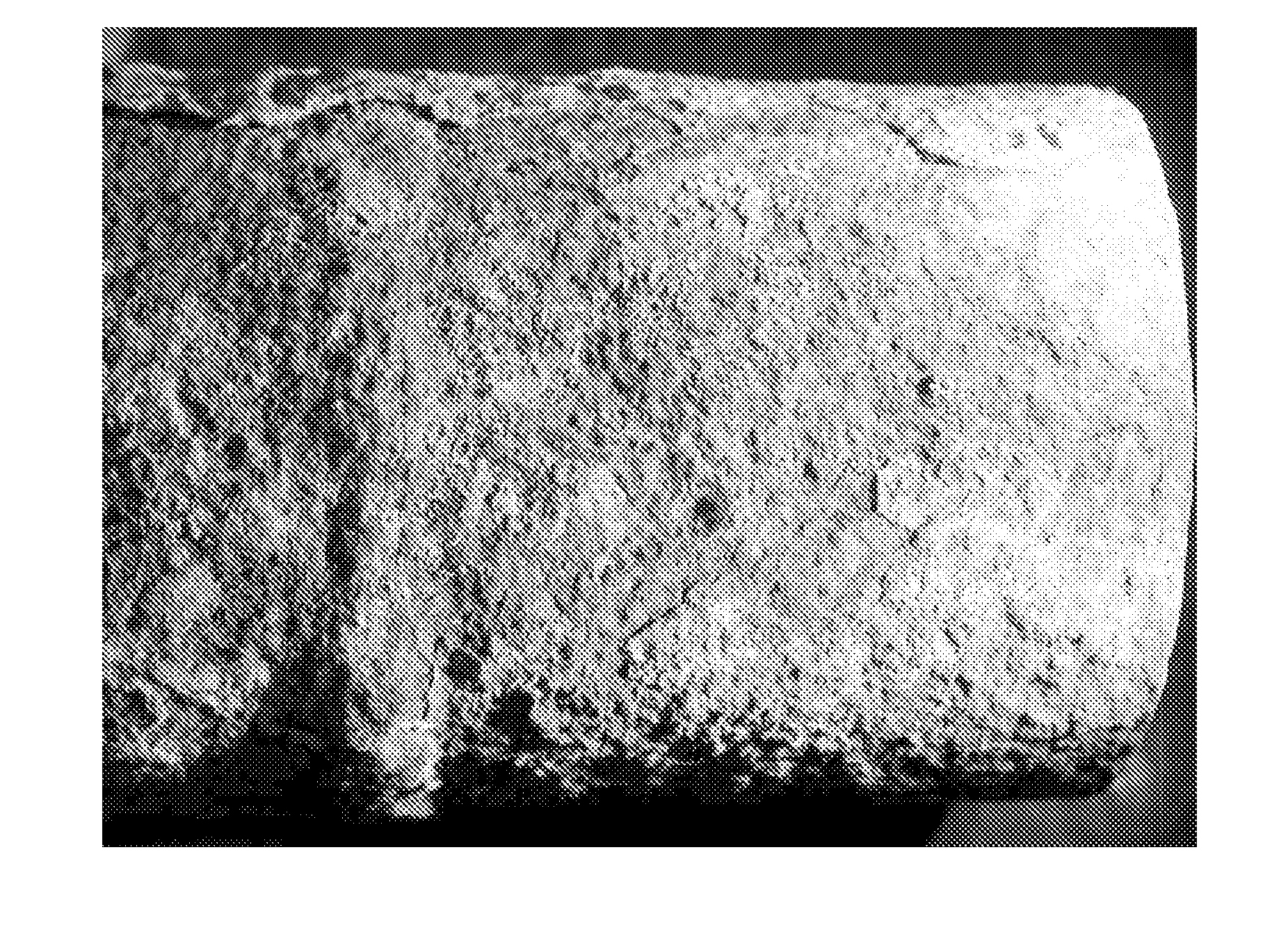
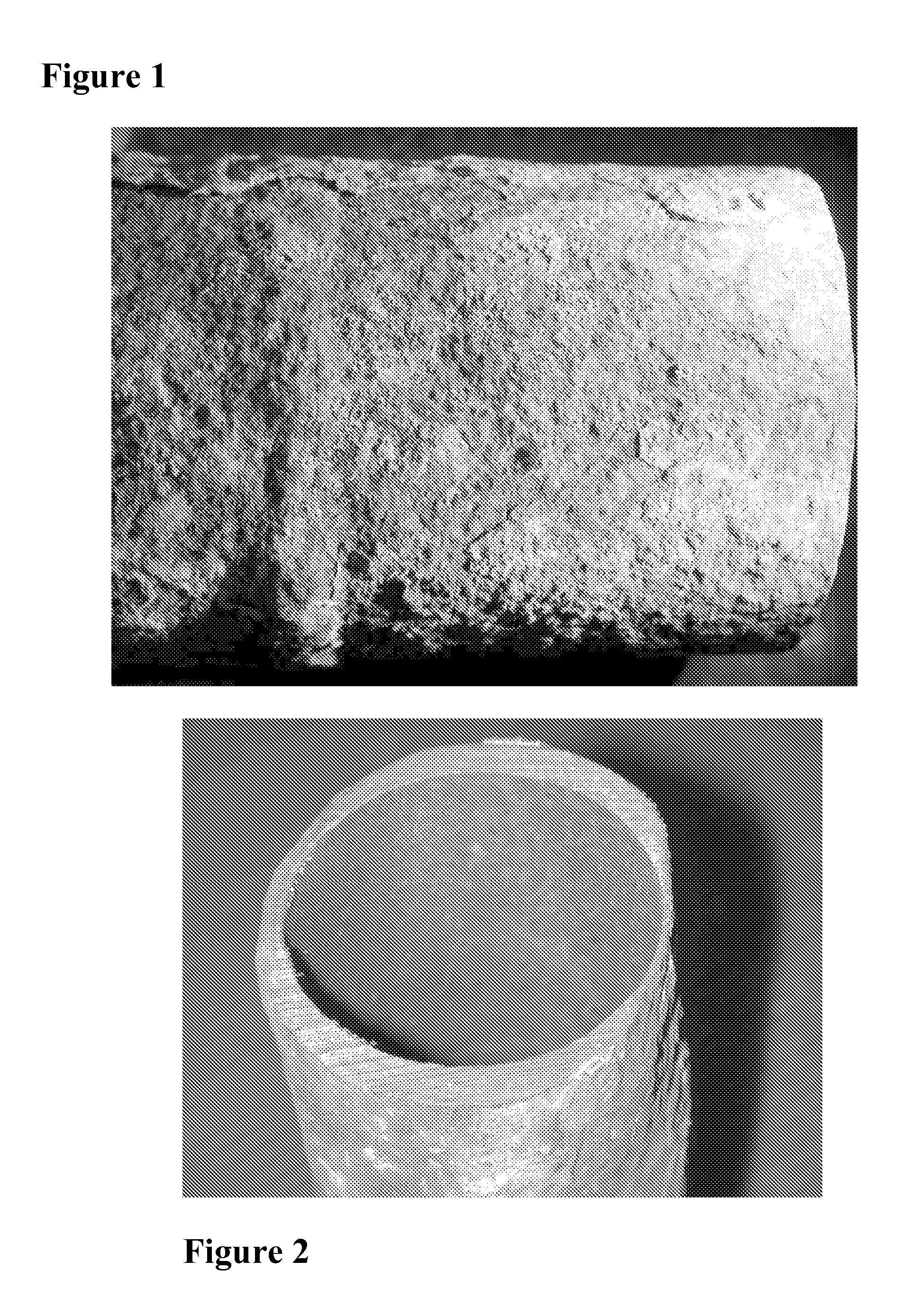
[0041] As discussed above, the problem of high temperature corrosion within waste to energy boilers by chlorides is one of the most costly in the industry. The invention enables assessment and control of corrosion of high temperature surfaces and preferably involves the controlled introduction of treatment chemicals by targeted in furnace injection to reduce corrosion while maximizing combustion efficiency. To illustrate the problem of chloride caused corrosion, applicants provide FIG. 1, which is a photograph showing a close-up of rough, wasted surface of a superheat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| corrosion rates | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com