Gluconobacter oxydans 2-ketoreductase enzyme and applications thereof
a technology of gluconobacter oxydans and ketoneductase, applied in the field of 2ketoneductase, can solve the problem that the large-scale synthesis of s-(+)-2-pentanol requires a large cell mass
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiments
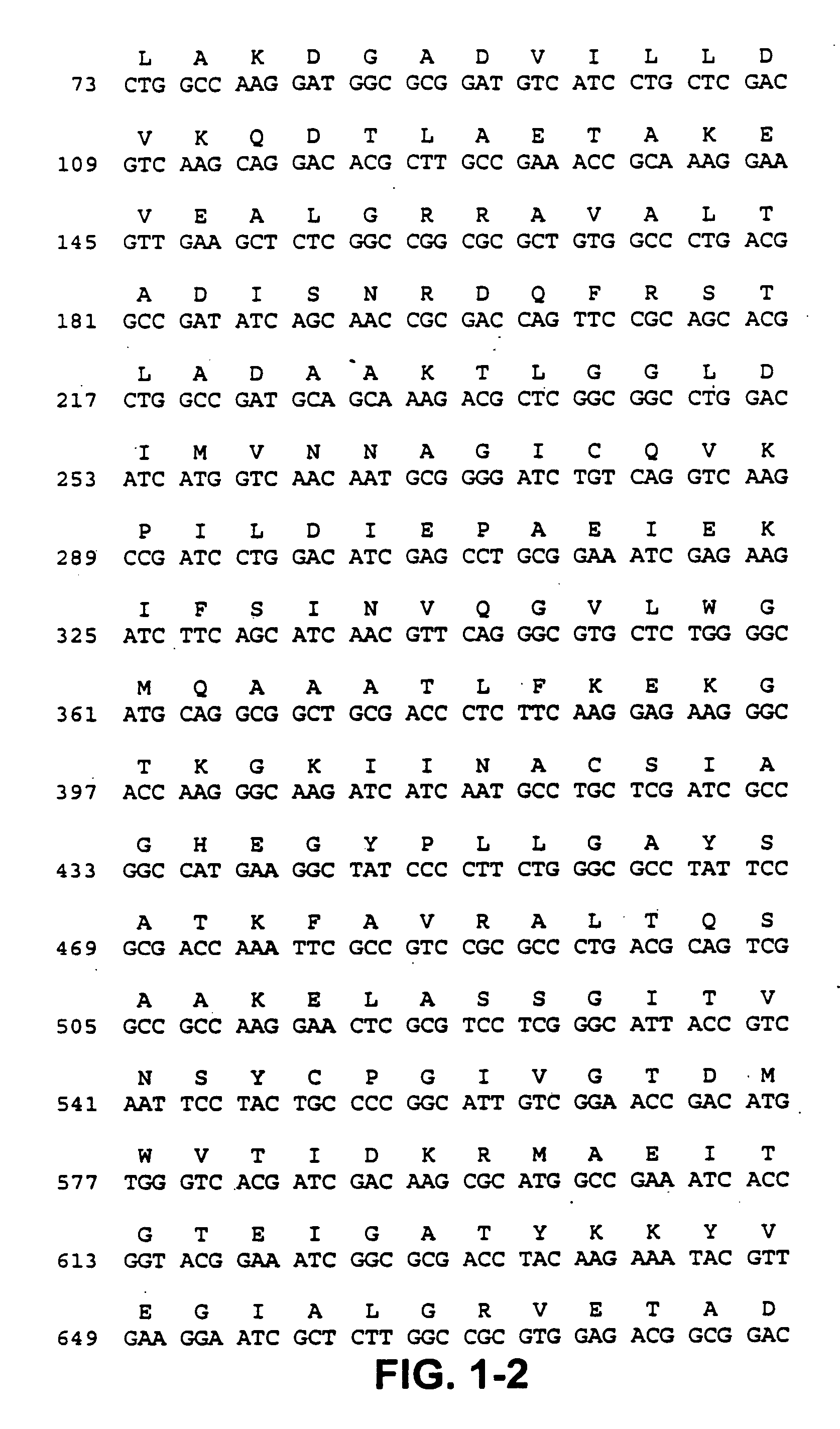
[0121] This invention encompasses, but is not limited to, the following embodiments: [0122] An isolated nucleic acid comprising a nucleotide sequence encoding amino acid sequence SEQ ID NO:2. [0123] An isolated nucleic acid comprising a nucleotide sequence encoding at least 12 contiguous residues of amino acid sequence SEQ ID NO:2. [0124] An isolated nucleic acid comprising a nucleotide sequence encoding at least 12 contiguous residues of the short chain dehydrogenase domain of amino acid sequence SEQ ID NO:2. [0125] An isolated nucleic acid comprising nucleotide sequence SEQ ID NO:1. [0126] An isolated nucleic acid comprising at least 21 contiguous nucleotides of nucleotide sequence SEQ ID NO:1. [0127] An isolated nucleic acid comprising a nucleotide sequence which is at least 54% identical to nucleotide sequence SEQ ID NO:1. [0128] An isolated nucleic acid comprising a nucleotide sequence which is complementary to a nucleotide sequence of the invention (above). [0129] A vector com...
example 1
Purification of G. Oxydans 2-Ketoreductase
[0183] Fermentation: Gluconobacter oxydans (SC13851) was grown on a glycerol-containing medium as follows. Cultures were grown in 500 ml Erlenmeyer flasks for 24 hr in 100 ml medium A (5% glycerol, 0.5% yeast extract, 0.05% ammonium sulfate, 0.3% peptone, 0.05% K2HPO4, 0.02% MgSO4.7H2O, 0.001% NaCl, 0.001% FeSO4.7H2O, and 0.001% MnSO4.7H2O). After 24 hr, the flask cultures were used to inoculate (1% v / v inoculum) a 15 L fermentor containing medium A. The fermentation was carried out at 28° C. for 24 hr. A 4000 L fermentor (Expend Industries, Inc., Brooklyn, N.Y.) was inoculated with 10 L inoculum from the 15 L fermentor. The 4000 L fermentor contained medium A with 0.05% antifoam SAG 5693. The fermentor was operated at 28° C., 100 LPM airflow, 690 mbar pressure, and 620 rpm agitation for 48 hr.
[0184] Cell recovery: The fermentor broth was cooled to 8° C. at the harvest. The tank was pressurized to 15 psig and broth was diverted to a Sharpl...
example 2
Analysis of Purified G. Oxydans 2-Ketoreductase
[0189] Protein assay: The Bio-Rad protein assay was used to determine protein concentration. The assay was performed according to the manufacturer's protocol (Bio-Rad). Samples containing 1-10 μl of enzyme fraction were brought to a volume of 0.8 ml with water. Next, 0.2 ml of the Bio-Rad reagent was added to the 0.8 ml sample solution. This was mixed thoroughly. The absorbance of the solution was measured at 595 nm. The protein concentration (mg / ml) was calculated from the standard curve using bovine serum albumin as standard protein.
[0190] Enzyme activity units: One unit (U) of enzyme activity was defined as one micromole of S-2-pentanol formed in 1 hr under the conditions described above. Results from the protein analysis of G. oxydans 2-ketoreductase are summarized below.
TABLE 1Enzyme ActivitySp. ActivityS-2-PentanolStepsVolume (mL)(Units)Protein (mg)(Units / mg)(e e)Purification FoldCell extract300390.00729.000.501.00DEAE Cellulo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com