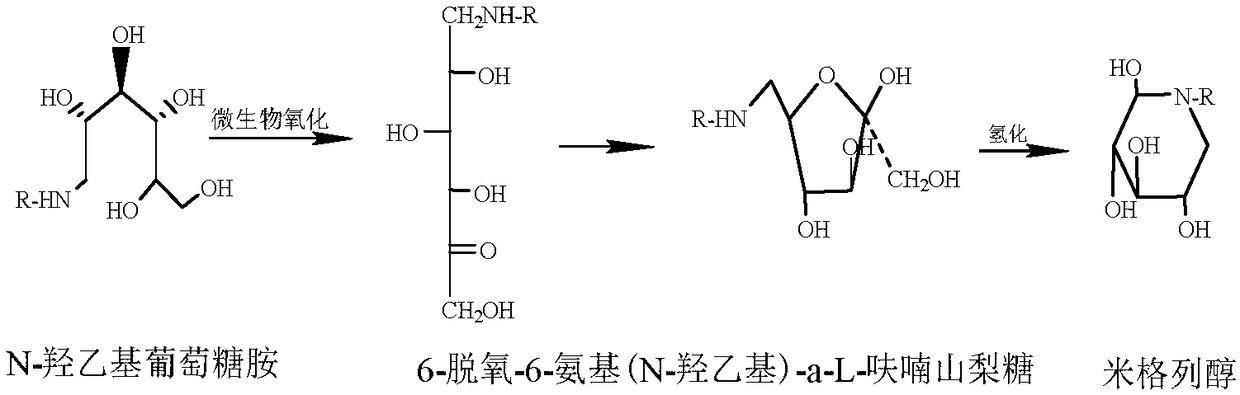
Method for rapidly screening mutant strain for synthesizing miglitol key intermediate, and strain thereof
A technology of miglitol and mutant strains, applied in the direction of microorganism-based methods, mutant preparation, biochemical equipment and methods, etc., can solve the problem of weak N-hydroxyethylglucosamine dehydrogenase activity, no rapid 6NSL Quantitative detection, limiting the production level of miglitol, etc., to achieve the effect of accelerating the mutagenesis selection process, good genetic stability, and increasing the amount of screening
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
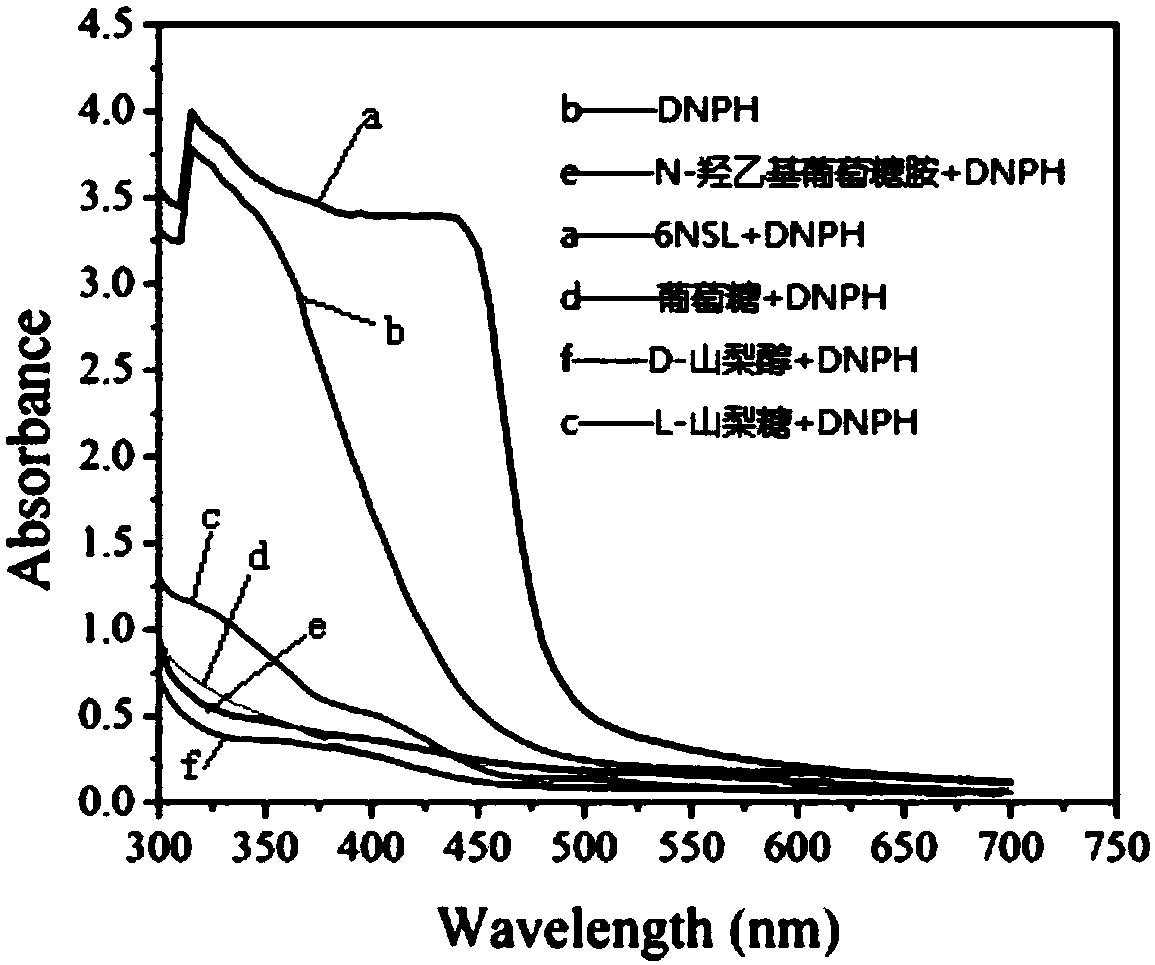
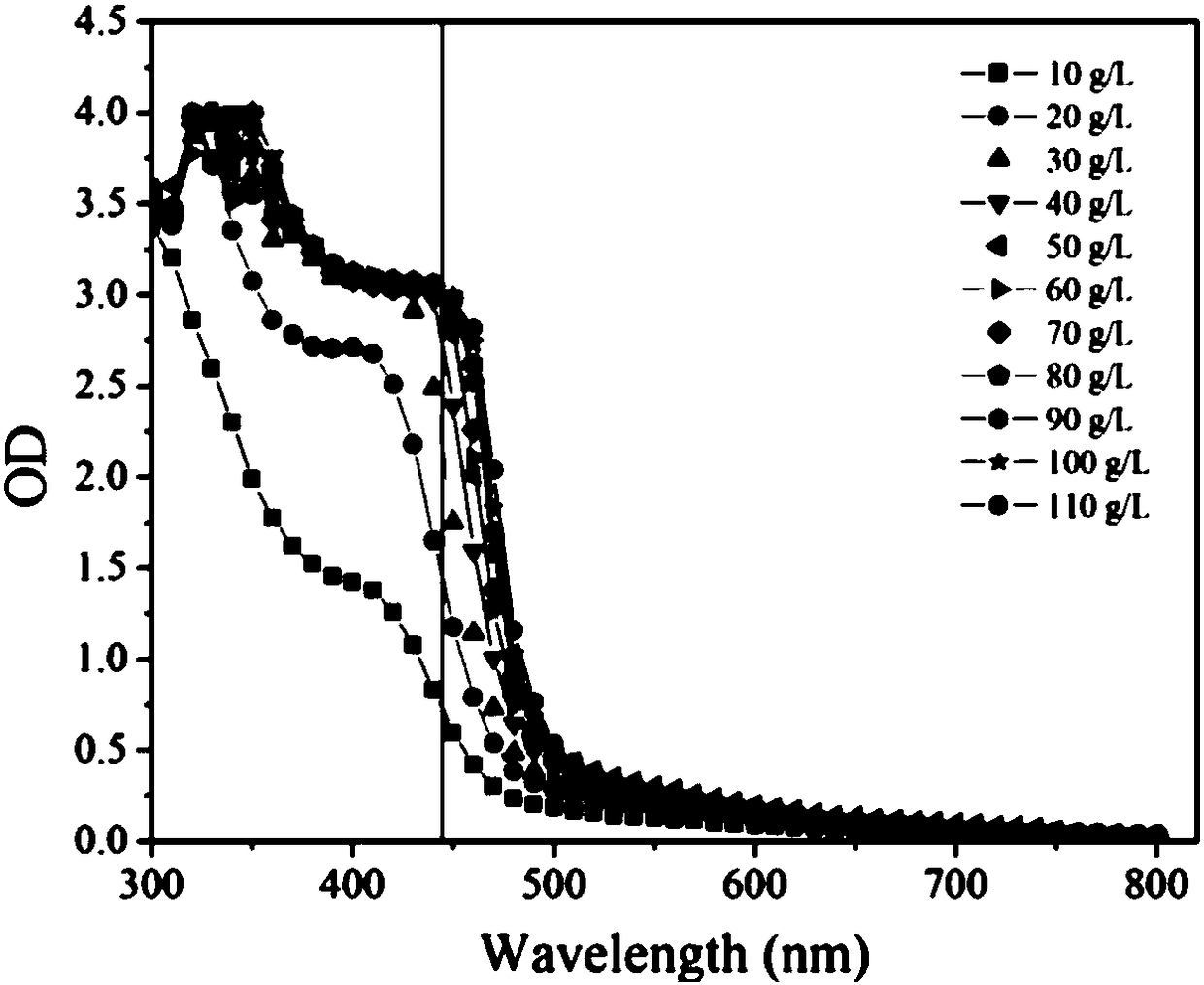
[0035] Example 1 Rapid Determination of Miglitol Key Intermediate 6NSL Chromogenic Method
[0036] (1) Preparation of reagents:
[0037] Preparation of DNPH chromogenic solution (20mM): Weigh 39.6mg of AR grade DNPH (dinitrophenylhydrazone), add 10mL of 10M HCl aqueous solution to completely dissolve DNPH, dilute to 100mL with deionized water, and transfer the solution to the brown reagent In the bottle, keep away from light.
[0038] Preparation of NaOH aqueous solution (8M): Weigh 32 g of NaOH solid and dissolve it in 100 mL of deionized water.
[0039] Preparation of the substrate reaction solution: N-hydroxyethylglucamine 60g / L and magnesium sulfate heptahydrate 5g / L, concentrated hydrochloric acid to adjust the pH to 5.0, and the solvent is deionized water.
[0040] (2) Determination steps: Pipette 150 μL of the substrate reaction solution into a 96-well plate, add 25 μL, 20 mM DNPH chromogenic solution for thorough mixing, keep the temperature at 37 ° C for 15 min, add...
Embodiment 2
[0056] Example 2 Mutation Breeding and High-throughput Screening of Gluconobacter oxidans
[0057] (1) Strain activation: Connect Gluconobacter oxydans (Gluconobacter oxydans ZJB-605CCTCC No.M 208069, Patent Publication No. CN101591681) from a glycerol tube to the slant medium, and cultivate it in a constant temperature incubator at 28°C for 3 to 5 days; The mass final concentration composition of the matrix is the same as in Example 1.
[0058] (2) Preparation of bacterial suspension and single spores: add 10mL 0.85% normal saline to the slant medium in step (1), scrape off the bacterial cells, pour the bacterial liquid into the Erlenmeyer flask with glass beads, shake After 10 minutes, the bacterium was dispersed, filtered into a sterilized empty Erlenmeyer flask with sterilized filter paper, and the filtered bacterium solution was diluted with sterile water, and the dilution factor was 10 -3 , which is the bacterial suspension;
[0059] (3) Ultraviolet mutagenesis: pipe...
Embodiment 3
[0072] Example 3 Comparison of the ability of Gluconobacter oxydans mutagen strain ZJB16009 and wild strain ZJB-605 to catalyze the synthesis of miglitol intermediate 6NSL
[0073] (1) Preparation of resting cells of Gluconobacter oxidans: Inoculate the slant medium (same as Example 1) with the starting strain Gluconobacter oxidans ZJB-605 and the mutagenized strain ZJB16009, cultivate at 28°C for 3 to 5 days, and wait for the slant culture CaCO 3 It becomes transparent and bright, and there is no bacteria in the naked eye. Pick the strains cultivated on the slant, inoculate the strains on the slant into a 250mL Erlenmeyer flask with 40mL seed culture solution (composition is the same as in Example 1), cultivate at 28°C and 235rpm for 48h, and obtain the seed solution, pH 4.0~7.0, OD 600 ≥8 reached the transition standard, and the microscopic examination showed short rod-shaped bacteria, dark coloring, and no miscellaneous bacteria. 2% inoculum by volume was transferred to t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com