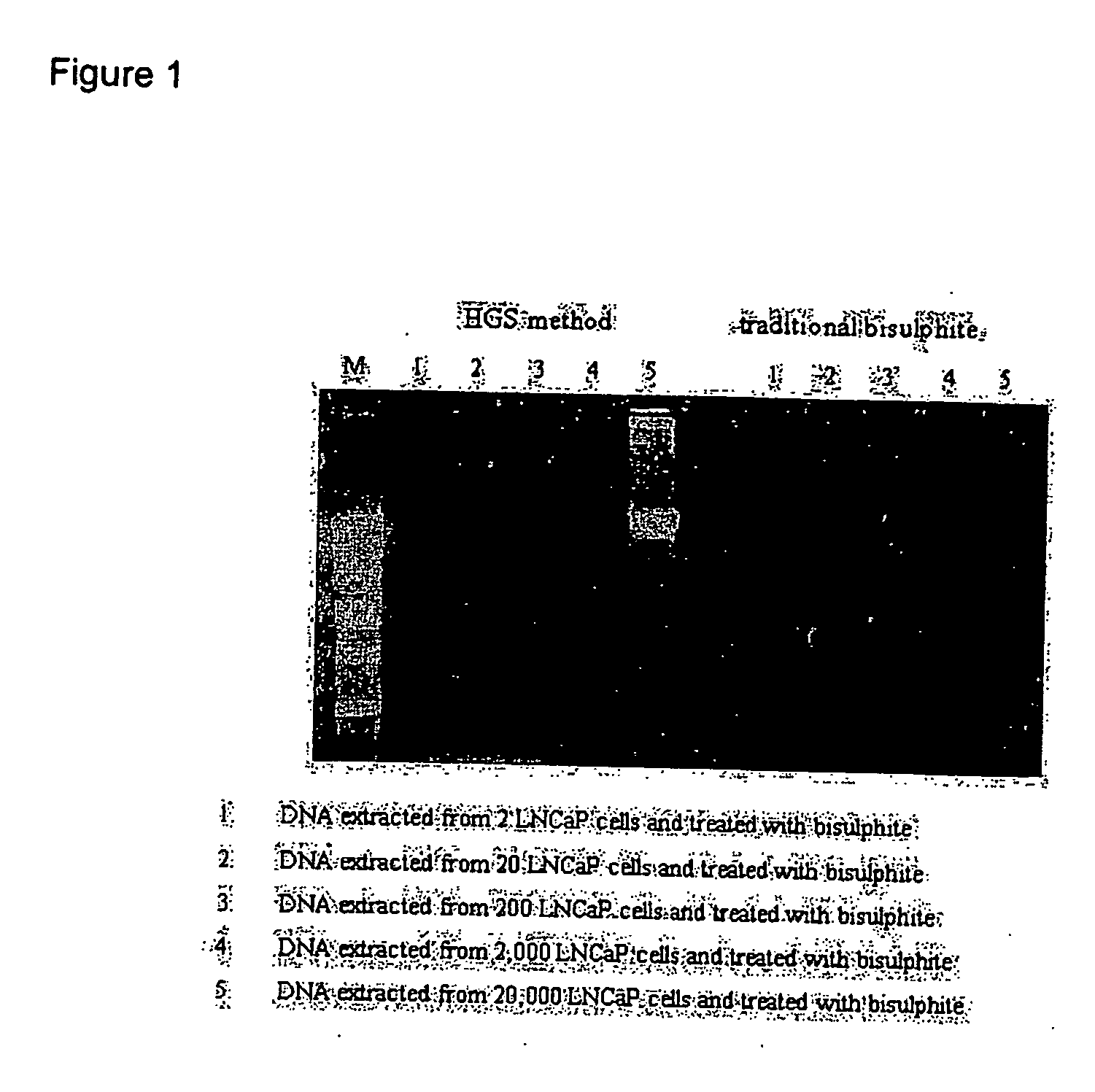
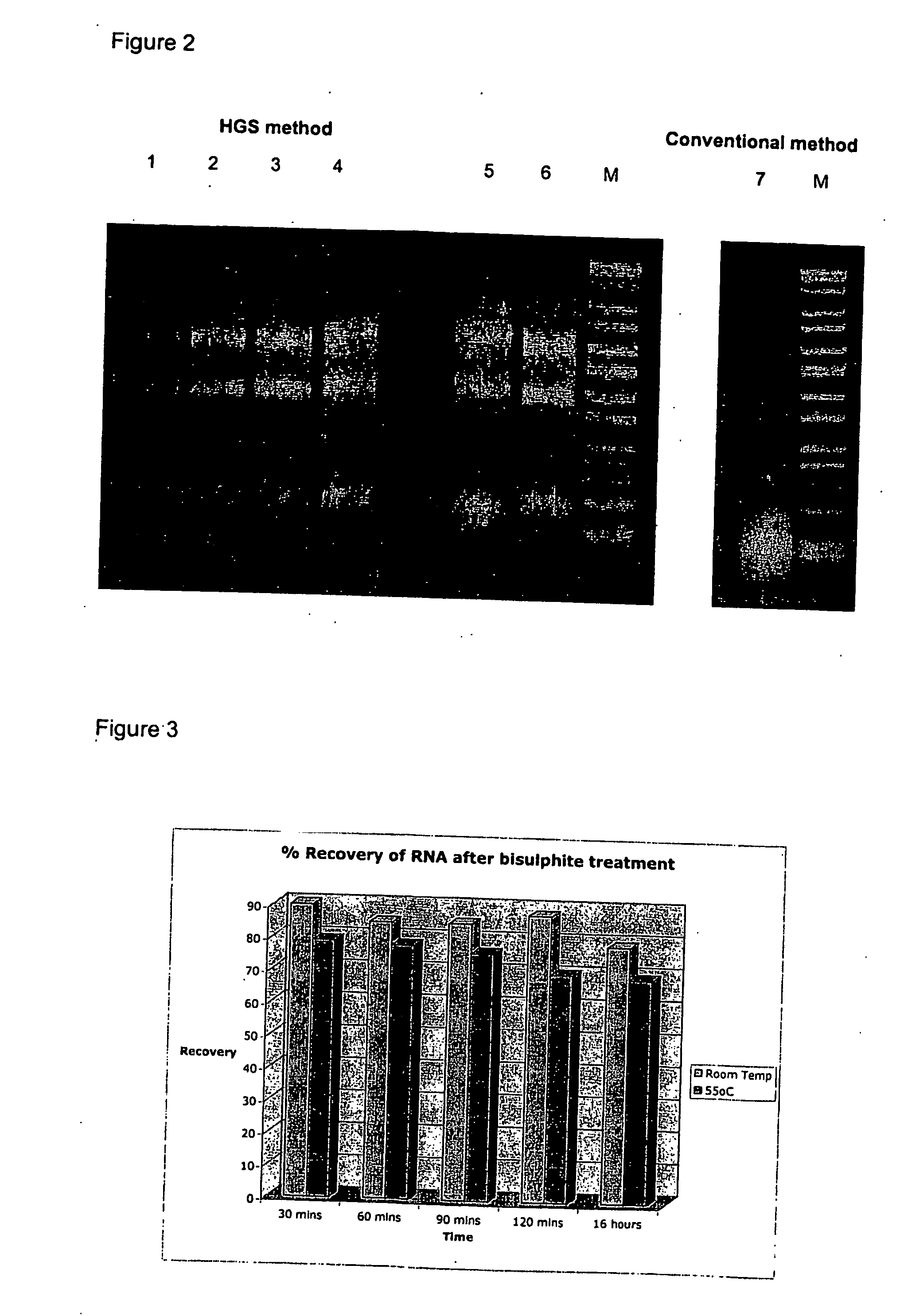
Treatment of nucleic acid
a nucleic acid and nucleic acid technology, applied in the field of nucleic acid treatment, can solve the problems of loss of up to about 96% of the nucleic acid sample, and about 4% of the nucleic acid is actually available for analysis, and achieve the effect of minimizing the loss of the sampl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Methods and Reagents
[0082] Chemicals were obtained as follows: Agarose from BioRad (Hercules Calif.; certified molecular biology grade #161-3101); Acetic acid, glacial, from BDH (Kylsyth, Australia; AnalaR 100015N); ethylenediamine tetraacetic acid (EDTA) from BDH (AnalaR 10093.5V); Ethanol from Aldrich (St. Louis Mo.; 200 proof E702-3); Isopropanol from Sigma (St. Louis Mo.; 99%+Sigma I-9516); Mineral oil from Sigma (M-5904); Sodium acetate solution 3M from Sigma (S-7899); Sodium chloride from Sigma (ACS reagent S9888); and Sodium hydroxide from BDH (AnalaR #10252.4X).
[0083] Enzymes / Reagents were obtained as follows: EcoR1 from Roche (Indianapolis Ind.; #87930626, 10 units / μl); HindIII from Biolabs (Beverly Mass.; #R01045, 10 units / μl); PCR master mix from Promega (Madison Wis; #M7505); and DNA markers from Sigma (Direct load PCR low ladder 100-1000 bp, Sigma D-3687 and 100-10 Kb, Sigma D-7058).
[0084] Solutions were as follows: (1) 10 mM Tris / 0.1M EDTA, pH 7.0-12.5; (2) 3M NaOH...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com