Fine particulate silver powder and production method thereof
a technology of production method, which is applied in the direction of coatings, etc., can solve the problems of significant factor of decreasing product yield, achieve low impurity content, achieve high dispersibility, and efficiently obtain fine particulate silver powder
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
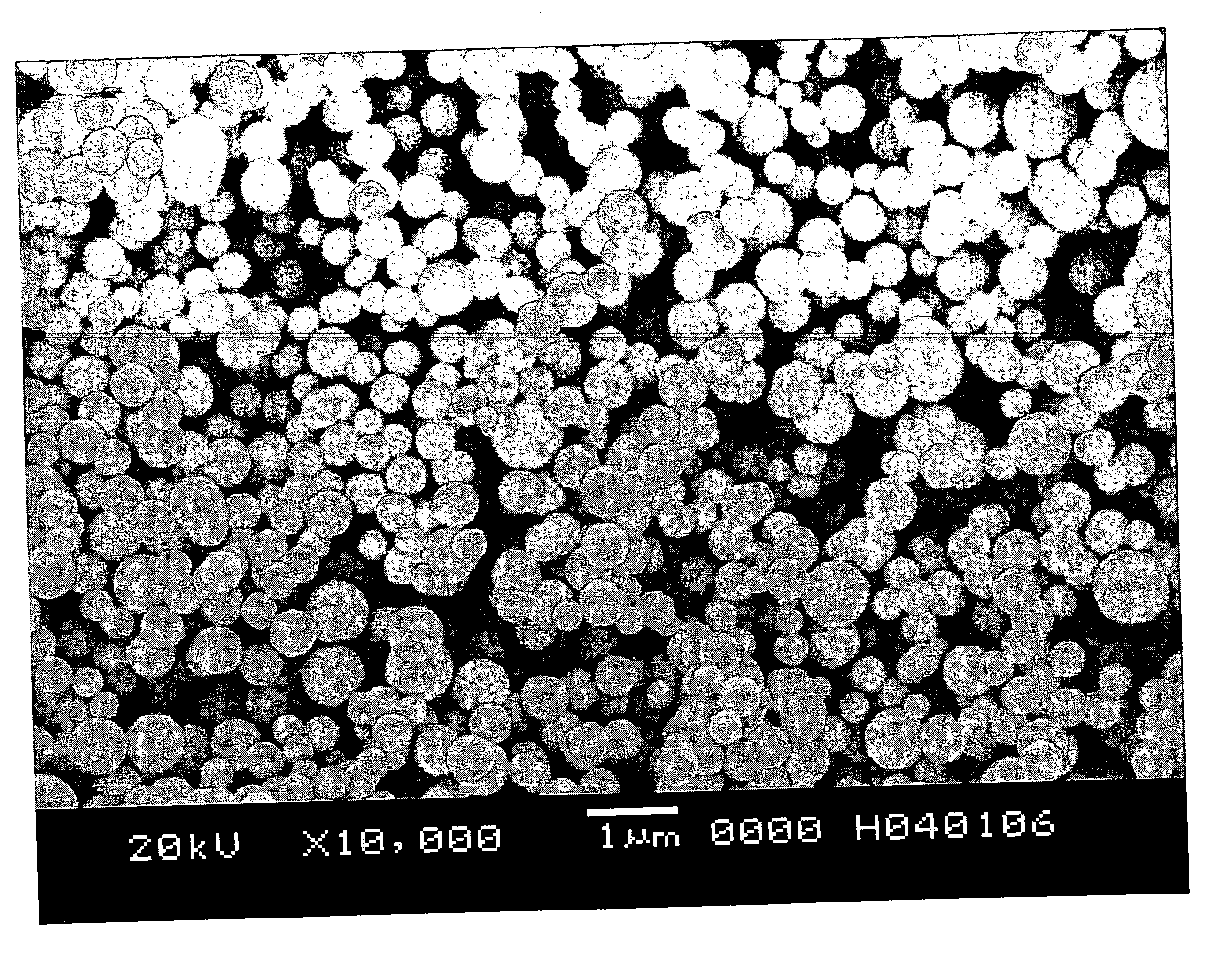
[0041] In this example, a fine particulate silver powder was produced using the production method stated above and the properties of the obtained fine particulate silver powder were measured. And further, a silver paste was produced with the fine particulate silver powder and a test circuit was formed and the conductor resistance and sintering starting temperature were measured.
[0042] First, 63.3 g of silver nitrate was dissolved in 9.7 liters of pure water to prepare a silver nitrate aqueous solution, and 235 ml of 25 wt % concentration ammonia water was added thereto at once and agitated and a silver ammine complex aqueous solution was obtained.
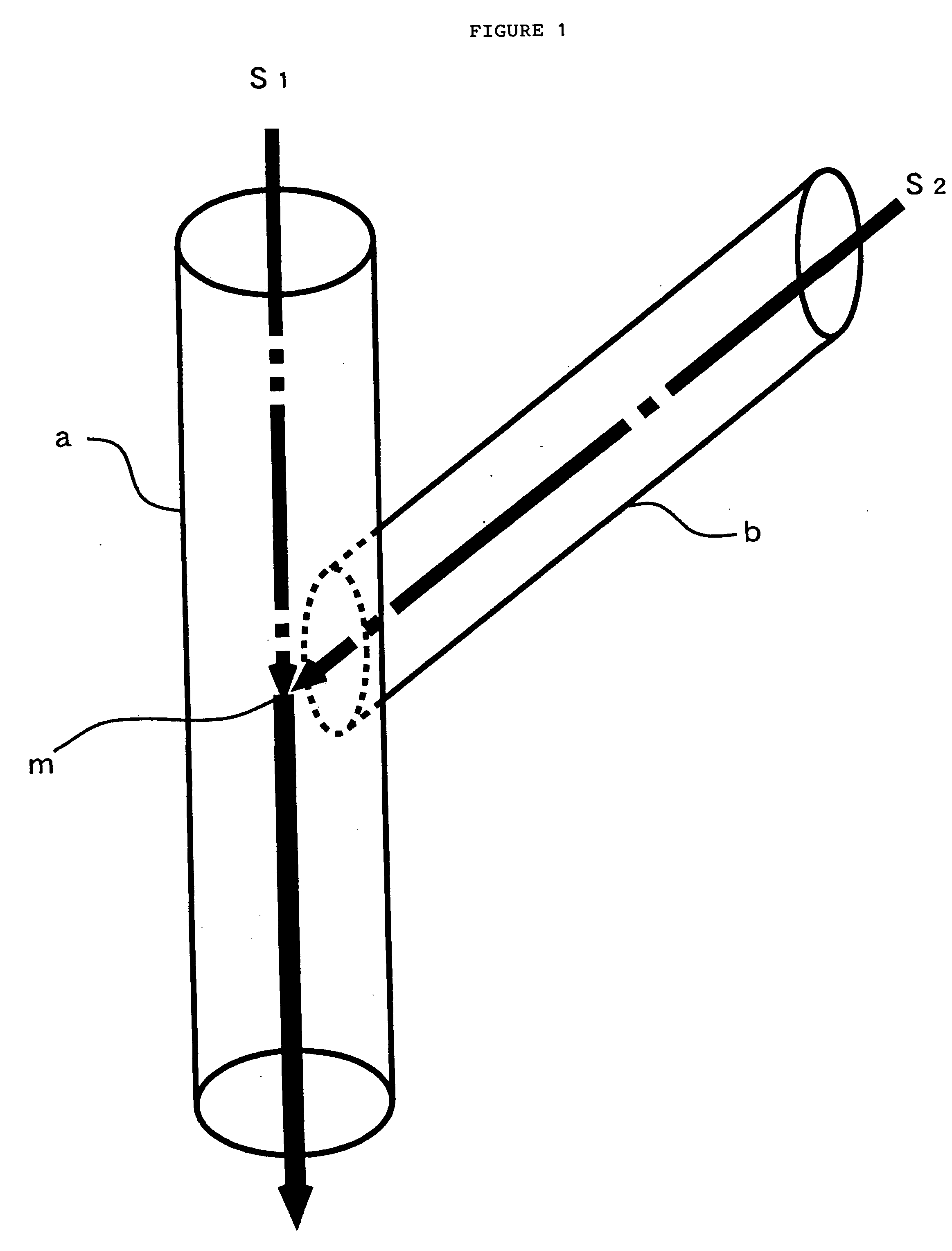
[0043] This silver ammine complex aqueous solution was introduced into the first flow path a of 13 mm inside diameter shown in FIG. 1 at a flow rate of 1,500 ml / sec and a reducing agent was flowed from the second flow path b at a flow rate of 1,500 ml / sec, and they were contacted at the joining point m at a temperature of 20° C. and a fin...
example 2
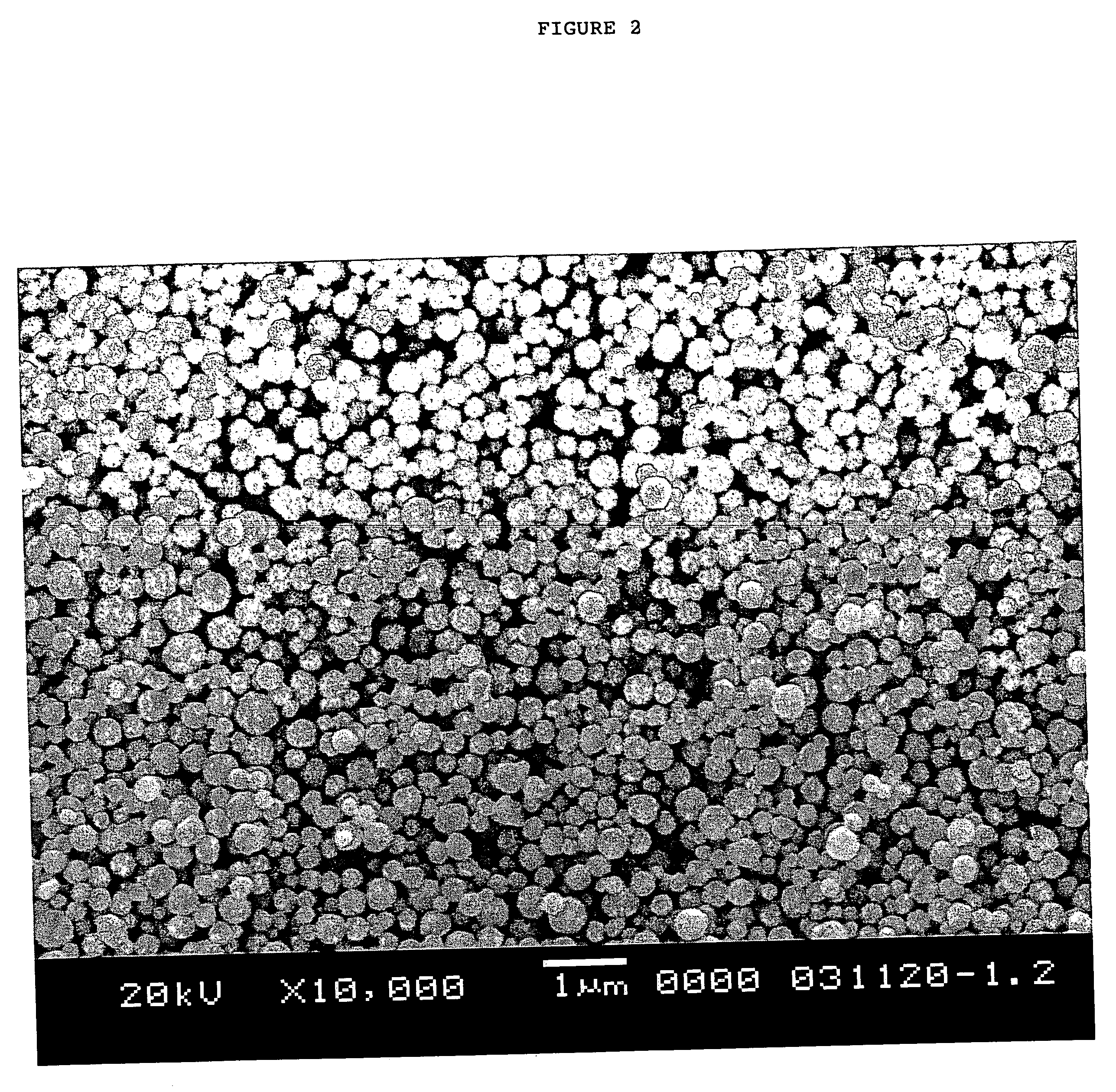
[0046] In this example, a fine particulate silver powder was produced using the production conditions different from those of Example 1 and the properties of the obtained fine particulate silver powder were measured. And further, a silver paste was produced with the fine particulate silver powder and a test circuit was formed and the conductor resistance and sintering starting temperature were measured.
[0047] First, 63.3 g of silver nitrate was dissolved in 3.1 liters of pure water to prepare a silver nitrate aqueous solution, and 235 ml of 25 wt % concentration ammonia water was added thereto at once and agitated and a silver ammine complex aqueous solution was obtained.
[0048] This silver ammine complex aqueous solution was introduced into the first flow path a of 13 mm inside diameter shown in FIG. 1 at a flow rate of 1,500 ml / sec and a reducing agent was flowed from the second flow path b at a flow rate of 1,500 ml / sec, and they were contacted at the joining point m at a temper...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com