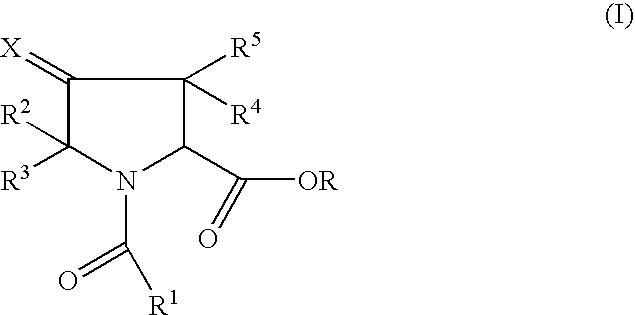
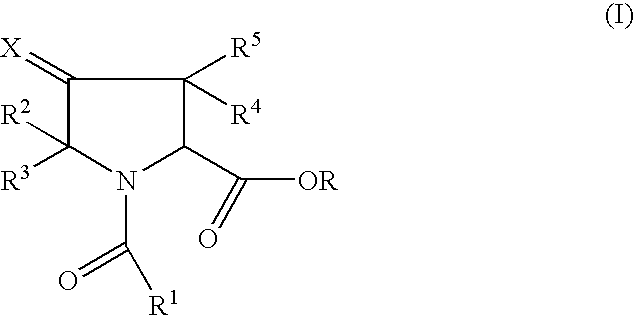
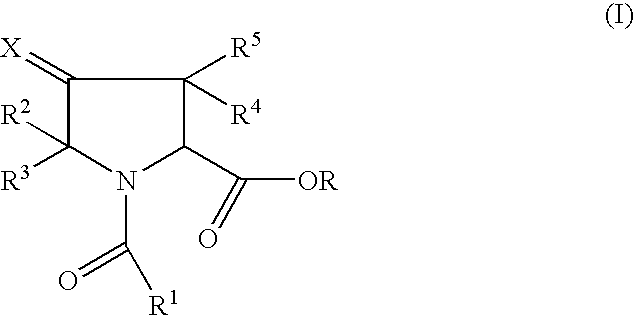
Pharmaceutically active pyrrolidine ester derivatives
a technology of pyrrolidine and ester, which is applied in the field of pyrrolidine esters, can solve the problems of significant problems in the management of preterm labor, undesired prematurity birth, and perinatal morbidity and mortality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
General Procedure for the Saponification of Methylesters of Oximether and / or Olefin-type 2-pyrrolidinecarboxylic Acid Intermediates (Schemes 3, 7)
[0136] A solution of sodium hydroxide (73 mg, 1.81 mmol) in water (1.2 ml) was added to a proline oximether methyl ester derivative, e.g. methyl (2S,4EZ)-4-(methoxyimino)-1-[(2′-methyl[1,1′-biphenyl]-4-yl)carbonyl]-2-pyrrolidinecarboxylate (391 mg, 1.1 mmol) in 3:1 dioxane:water (12 ml) and the reaction stirred for 3 h. The reaction mixture was then washed with diethyl ether (2×10 ml), and the aqueous phase acidified to pH 2 (0.1N HCl) and extracted into ethyl acetate. The ethyl acetate layer was then dried over magnesium sulfate, filtered and the solvent was then removed in vacuo to give the desired product, e.g. (2S4EZ)-4-(methoxyimino)-1-[(2′-methyl[1,1′-biphenyl]-4-yl)carbonyl]-2-pyrrolidine-carboxylic acid in 91% yield as an oil which was used without further purification.
[0137]1H NMR (300 MHz, CDCl3): 2.25 (m, 3H, ArCH3), 2.96-3.3...
example 2
General Protocols for the Esterification of Oximeter- and / or Olefin-type 2-pyrrolidinecarboxylic Acid Intermediates (Schemes 2, 5, 7):
a) Methylesters (e.g. 1-tert-butyl 2-methyl (2S,4EZ)-4-(methoxyimino)-1,2-pyrrolidine-dicarboxylate):
[0138] A solution of the oximether- and / or olefin-type 2-pyrrolidinecarboxylic acid intermediate, e.g. (2S,4EZ)-1-(tert-butoxycarbonyl)-4-(methoxyimino)-2-pyrrolidinecarboxylic acid (0.648 g, 2.5 mmol), in a 1:1 mixture of methanol and toluene (35 ml) was made. Trimethylsilyl diazomethane (3.8 ml of a 2M solution in hexanes, 7.5 mmol) was then added dropwise to the stirred solution at room temperature under nitrogen. After completion of the evolution of nitrogen gas, the resulting yellow solution was evaporated in vacuo, and the residue filtered through a pad of silica gel, eluting with ethyl acetate. Removal of solvent from the filtrate gave the methylester product, e.g. 1-tert-butyl 2-methyl (2S,4EZ)-4-(methoxyimino)-1,2-pyrrolidinedicarboxylate,...
example 3
Cyclopentyl (2S,4EZ)-4-(methoxyimino)-1-[(2′-methyl[1,1′-biphenyl]-4-yl)carbonyl]-2-pyrrolidinecarboxylate
[0141] Following the general methods as outlined in Example 2, starting from (2S,4EZ)-4-(methoxyimino)-1-[(2′-methyl[1,1′-biphenyl]-4-yl)carbonyl]-2-pyrrolidinecarboxylic acid and cyclopentanol, the title compound was isolated, after flash-chromatography, as a mixture of two isomers as an oil in 57% yield (95.6% purity by HPLC).
[0142]1H NMR (300 MHz, CDCl3): 1.47-1.98 (m, 8H), 2.24 (m, 3H, ArCH3), 2.73-3.14 (m, 2H), 3.84 (m, 3H, NOCH3), 4.11-4.46 (m, 2H), 4.61 (br s, 1H), 4.99-5.32 (m, 2H), 7.15-7.28 (m, 4H), 7.31-7.41 (m, 2H), 7.51-7.62 (m, 2H). M+(APCI+): 421.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com